Asymmetric (stereoselective) synthesis
... products as starting materials • Enalapril is prepared by a diastereoselective reductive amination between ethyl-2-oxo-4-phenylbutyrate and alanyl-proline favoring the desired (S,S,S)-enantiomer (17:1) ...
... products as starting materials • Enalapril is prepared by a diastereoselective reductive amination between ethyl-2-oxo-4-phenylbutyrate and alanyl-proline favoring the desired (S,S,S)-enantiomer (17:1) ...
Microwave-Enhanced Sulphated Zirconia and SZ/MCM
... Synthesis of sulphated zirconia supported on MCM-41 Preparation of the sulphated zirconia supported on MCM-41 (SZM) has been already described. [20] Siliceous MCM-41 (300 mg) were mixed with zirconium sulphate (15 mL) in methanol (1 wt % S), the heterogeneous mixture was stirred 14 h. Then, the impr ...
... Synthesis of sulphated zirconia supported on MCM-41 Preparation of the sulphated zirconia supported on MCM-41 (SZM) has been already described. [20] Siliceous MCM-41 (300 mg) were mixed with zirconium sulphate (15 mL) in methanol (1 wt % S), the heterogeneous mixture was stirred 14 h. Then, the impr ...
Grignard Reaction - This is Synthesis
... Efficient Grignard Reaction under Sealed Vessel Conditions The Grignard reaction is a powerful, frequently used organometallic transformation to add important structural motifs to carbonyl compounds resulting in secondary or tertiary alcohols. Since usually the low boiling THF is used as a solvent, ...
... Efficient Grignard Reaction under Sealed Vessel Conditions The Grignard reaction is a powerful, frequently used organometallic transformation to add important structural motifs to carbonyl compounds resulting in secondary or tertiary alcohols. Since usually the low boiling THF is used as a solvent, ...
Alkenes undergo Addition Reactions Predict the product of each
... 1. Write the structure of the principal organic product from the reaction of 1-bromopropane with sodium acetate (CH3COONa) in acetic acid. 2. Outline an efficient synthesis of the following compound from the indicated starting material and any necessary organic or inorganic reagents: cyclopentyl cy ...
... 1. Write the structure of the principal organic product from the reaction of 1-bromopropane with sodium acetate (CH3COONa) in acetic acid. 2. Outline an efficient synthesis of the following compound from the indicated starting material and any necessary organic or inorganic reagents: cyclopentyl cy ...
PART 3 Principles and Applications of Organometallics in Catalysis
... In the OA we break the (2-electron) A—B bond and form two (2-electron) bonds to the metal, i.e. M—A and M—B. Hence, there are some requirements for OA to occur. (i) A vacant coordination site must be available on the metal complex, and (ii) the metal must have an accessible oxidation state 2 units h ...
... In the OA we break the (2-electron) A—B bond and form two (2-electron) bonds to the metal, i.e. M—A and M—B. Hence, there are some requirements for OA to occur. (i) A vacant coordination site must be available on the metal complex, and (ii) the metal must have an accessible oxidation state 2 units h ...
Exam only.
... 1. This exam contains 17 pages of questions and instructions, a page of equations, a list of standard reduction potentials, a periodic table, and a set of constants and conversion factors. 2. There are 44 multiple choice questions. Circle the one best answer for each. 3. You have 120 minutes to work ...
... 1. This exam contains 17 pages of questions and instructions, a page of equations, a list of standard reduction potentials, a periodic table, and a set of constants and conversion factors. 2. There are 44 multiple choice questions. Circle the one best answer for each. 3. You have 120 minutes to work ...
Hydrogen Production by Splitting Water in an Electrolyzer
... Studies of reacting systems - whether small scale reactions in a laboratory or large scale processes, all focus on obtaining a basic description of the individual steps involved in the reaction, and the characteristic rate of each step. In most studies, this information is obtained by experimental a ...
... Studies of reacting systems - whether small scale reactions in a laboratory or large scale processes, all focus on obtaining a basic description of the individual steps involved in the reaction, and the characteristic rate of each step. In most studies, this information is obtained by experimental a ...
Part B: Short Written Response - bourre-chem-11
... a) Fractions that have higher boiling points settle at the bottom, and fractions with lower boiling points settle to the bottom. b) Fractions that have lower boiling points settle at the bottom, and fractions with higher boiling points settle to the bottom. c) The boiling point depends on the length ...
... a) Fractions that have higher boiling points settle at the bottom, and fractions with lower boiling points settle to the bottom. b) Fractions that have lower boiling points settle at the bottom, and fractions with higher boiling points settle to the bottom. c) The boiling point depends on the length ...
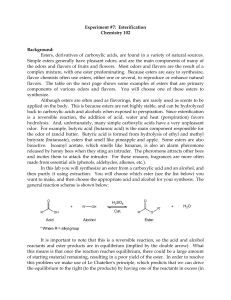
ESTERIFICATION
... Sulfuric acid (H2SO4) is used as a catalyst for this reaction in order to accelerate the rate at which the product is formed. Since a catalyst is not consumed during the course of a reaction, you need to use only a small amount of sulfuric acid in order for it to be effective. Heating is another way ...
... Sulfuric acid (H2SO4) is used as a catalyst for this reaction in order to accelerate the rate at which the product is formed. Since a catalyst is not consumed during the course of a reaction, you need to use only a small amount of sulfuric acid in order for it to be effective. Heating is another way ...
Naming Ethers Naming Ethers
... • Skunks use thiols as a defense mechanism: (E)-2butene-1-thiol, 3-methyl-1-butanethiol, and 2quinolinemethanethiol, and acetate thioesters of these. • Methanethiol is added to natural gas (methane) so that gas leaks can be detected. • The hydrosulfide ion (HS–) is a strong nucleophile and a weak ...
... • Skunks use thiols as a defense mechanism: (E)-2butene-1-thiol, 3-methyl-1-butanethiol, and 2quinolinemethanethiol, and acetate thioesters of these. • Methanethiol is added to natural gas (methane) so that gas leaks can be detected. • The hydrosulfide ion (HS–) is a strong nucleophile and a weak ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 PART-A
... ‘Aryldiazonium salt involves both in electrophilic and nucleophilic substitutions’. Justify. What is first order asymmetric transformation? Give an example. How do inductive and field effects affect the second substitution in aromatic systems? In 4-t-butylcyclohexanecarboxylic acid, trans form is mo ...
... ‘Aryldiazonium salt involves both in electrophilic and nucleophilic substitutions’. Justify. What is first order asymmetric transformation? Give an example. How do inductive and field effects affect the second substitution in aromatic systems? In 4-t-butylcyclohexanecarboxylic acid, trans form is mo ...
Lab 7_Esterification
... Sulfuric acid (H2SO4) is used as a catalyst for this reaction in order to accelerate the rate at which the product is formed. Since a catalyst is not consumed during the course of a reaction, you need to use only a small amount of sulfuric acid in order for it to be effective. Heating is another way ...
... Sulfuric acid (H2SO4) is used as a catalyst for this reaction in order to accelerate the rate at which the product is formed. Since a catalyst is not consumed during the course of a reaction, you need to use only a small amount of sulfuric acid in order for it to be effective. Heating is another way ...
Excercises 6-10
... 1. The following chemical transformation of 2-‐bromo-‐1-‐phenylpropane to (E)-‐1-‐ phenylprop-‐1-‐ene is an elimination reaction. These β-‐hydrogen eliminations can proceed via three different mechanisms. Please p ...
... 1. The following chemical transformation of 2-‐bromo-‐1-‐phenylpropane to (E)-‐1-‐ phenylprop-‐1-‐ene is an elimination reaction. These β-‐hydrogen eliminations can proceed via three different mechanisms. Please p ...
MS PowerPoint - Catalysis Eprints database
... A one-carbon synthon for alkylation of aromatics and active methylene derivatives Precursors to synthesize methyl ethers and Chloromethyl ether derivatives ...
... A one-carbon synthon for alkylation of aromatics and active methylene derivatives Precursors to synthesize methyl ethers and Chloromethyl ether derivatives ...
Energy and Reactions
... without showing the activation energy. It could also be seen as quite zero activation energy, but reactions between two ions of opposite charge usually has a very low activation energy. ...
... without showing the activation energy. It could also be seen as quite zero activation energy, but reactions between two ions of opposite charge usually has a very low activation energy. ...
Exam 1 Key
... calorimeter is filled with 4520 g of water and has a heat capacity for the hardware of 2.350 kJ/°C. Before ignition the temperature was 27.500°C. After ignition the temperature stabilized at 33.170°C. (a) Write out the entire balanced reaction for this combustion. Make sure you include the state of ...
... calorimeter is filled with 4520 g of water and has a heat capacity for the hardware of 2.350 kJ/°C. Before ignition the temperature was 27.500°C. After ignition the temperature stabilized at 33.170°C. (a) Write out the entire balanced reaction for this combustion. Make sure you include the state of ...
CHEMISTRY 1000
... Any bond can be hydrogenated; however, C=O bonds are stronger than C=C, C=N, CC or CN bonds, so hydrogenation of a carbonyl requires a more powerful catalyst than Pd. Draw the major organic product of each hydrogenation reaction: H2 Pd/C ...
... Any bond can be hydrogenated; however, C=O bonds are stronger than C=C, C=N, CC or CN bonds, so hydrogenation of a carbonyl requires a more powerful catalyst than Pd. Draw the major organic product of each hydrogenation reaction: H2 Pd/C ...
Notes 07 Organometallic Compounds with notes
... R2CuLi + R’-X R-R’ + RCu + LiX Reaction Type: Nucleophilic Substitution. Creation of new C-C bonds. 1 alkyl iodides are best, otherwise an elimination reaction can occur. The R’ group in the halide can be aryl or vinyl. The R group of the cuprate can be aryl or vinyl. Although the mechanism looks ...
... R2CuLi + R’-X R-R’ + RCu + LiX Reaction Type: Nucleophilic Substitution. Creation of new C-C bonds. 1 alkyl iodides are best, otherwise an elimination reaction can occur. The R’ group in the halide can be aryl or vinyl. The R group of the cuprate can be aryl or vinyl. Although the mechanism looks ...
Organic Functional Groups
... - polar region (less than carboxylic acids) - prepared by a reversible condensation reaction (usually an alcohol and c. acid) with removal of a small molecule such as water - reaction usually needs an acid catalyst and is sometimes called ...
... - polar region (less than carboxylic acids) - prepared by a reversible condensation reaction (usually an alcohol and c. acid) with removal of a small molecule such as water - reaction usually needs an acid catalyst and is sometimes called ...
CN>Chapter 22CT>Carbonyl Alpha
... The carbon next to the carbonyl group is designated as being in the position Electrophilic substitution occurs at this position through either an enolate or enol ion ...
... The carbon next to the carbonyl group is designated as being in the position Electrophilic substitution occurs at this position through either an enolate or enol ion ...
+ 2 O 2 - SandersScienceStuff
... • For products that are ionic compounds in water: use the solubility rules on the back of your periodic table to determine the state of matter. Insoluble substances will exist as solids. ...
... • For products that are ionic compounds in water: use the solubility rules on the back of your periodic table to determine the state of matter. Insoluble substances will exist as solids. ...
Lecture12
... - The reaction is considered to be a concerted process - The regiochemistry of the intermolecular Heck insertion step is highly sensitive to the electronics of the substrate, the reaction manifold, and steric congestion. As a result regioselectivity can be poor for certain classes of substrates - Th ...
... - The reaction is considered to be a concerted process - The regiochemistry of the intermolecular Heck insertion step is highly sensitive to the electronics of the substrate, the reaction manifold, and steric congestion. As a result regioselectivity can be poor for certain classes of substrates - Th ...
Remodeling of the natural product fumagillol
... conditions was evaluated. When 11 was reacted with several anilines, the regioselectivity obtained with La(III) catalysis inverted, leading predominantly to perhydroisoquinoline products as originally found in the Zn(II)-catalyzed reactions (Figure 2b). By comparison, reaction of 11 using Zn(II) bec ...
... conditions was evaluated. When 11 was reacted with several anilines, the regioselectivity obtained with La(III) catalysis inverted, leading predominantly to perhydroisoquinoline products as originally found in the Zn(II)-catalyzed reactions (Figure 2b). By comparison, reaction of 11 using Zn(II) bec ...
Ene reaction

The ene reaction (also known as the Alder-ene reaction) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Also,many useful Lewis acid-catalyzed ene reactions have been developed which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products.