A review of new developments in the Friedel–Crafts - Beilstein
... be utilized leading to vast amounts of salt side products. With the need for more environmentally and economically benign processes, the development of FC reactions using only catalytic amounts of a metal or acid catalyst would be highly desirable. In addition, the substitution of the alkyl chloride ...
... be utilized leading to vast amounts of salt side products. With the need for more environmentally and economically benign processes, the development of FC reactions using only catalytic amounts of a metal or acid catalyst would be highly desirable. In addition, the substitution of the alkyl chloride ...
Lecture - Ch 21
... – Acid chlorides are the most reactive because the electronegative chlorine withdraws electrons from the carbonyl carbon CHE2202, Chapter 21 – Amides are the least reactive Learn, 15 ...
... – Acid chlorides are the most reactive because the electronegative chlorine withdraws electrons from the carbonyl carbon CHE2202, Chapter 21 – Amides are the least reactive Learn, 15 ...
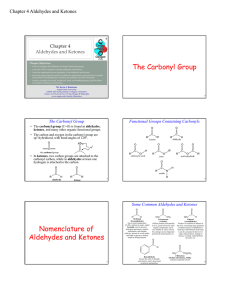
The Carbonyl Group Nomenclature of Aldehydes and Ketones
... • Since there is no hydrogen on the carbonyl oxygen, aldehydes and ketones do not form hydrogen bonds with themselves. • Aldehydes and ketones therefore have boiling points that are in between those of alcohols and hydrocarbons of the same molecular weight: – Alcohols form hydrogen bonds, and have h ...
... • Since there is no hydrogen on the carbonyl oxygen, aldehydes and ketones do not form hydrogen bonds with themselves. • Aldehydes and ketones therefore have boiling points that are in between those of alcohols and hydrocarbons of the same molecular weight: – Alcohols form hydrogen bonds, and have h ...
Chapter 27
... When D-galactose reacts to form a cyanohydrin, the carbonyl group reacts with HCN. This reaction is called addition because cyanide adds to the carbonyl carbon while H adds to the carbonyl oxygen. The double bond is lost in this process. Two isomers form in this ...
... When D-galactose reacts to form a cyanohydrin, the carbonyl group reacts with HCN. This reaction is called addition because cyanide adds to the carbonyl carbon while H adds to the carbonyl oxygen. The double bond is lost in this process. Two isomers form in this ...
INTRODUCTION - Open Access Repository of Indian Theses
... 3. A Mild and Highly Efficient Synthesis of 3-Pyrrolyl-Indolinones and Pyrrolyl-Indeno[1,2-b]Quinoxalines using BiCl3 as a Catalyst An efficient synthesis of 3-pyrrolyl-indolinones and pyrrolyl-indeno[1,2-b] quinoxalines is described by the reaction of 4-hydroxyproline with isatin or indeno[1,2b]qui ...
... 3. A Mild and Highly Efficient Synthesis of 3-Pyrrolyl-Indolinones and Pyrrolyl-Indeno[1,2-b]Quinoxalines using BiCl3 as a Catalyst An efficient synthesis of 3-pyrrolyl-indolinones and pyrrolyl-indeno[1,2-b] quinoxalines is described by the reaction of 4-hydroxyproline with isatin or indeno[1,2b]qui ...
Presence N-Methyl groups
... 1. Tropine possess a 7 membered carbon ring. 2. It contains a reduced pyridine ring in the structure 3. It possess reduced pyrrole (pyrollidine) ring in the structure 4. It possess N-Methyl group As tropine contains only one nitrogen atom it means that this should also remain common to pyrollidine a ...
... 1. Tropine possess a 7 membered carbon ring. 2. It contains a reduced pyridine ring in the structure 3. It possess reduced pyrrole (pyrollidine) ring in the structure 4. It possess N-Methyl group As tropine contains only one nitrogen atom it means that this should also remain common to pyrollidine a ...
Organic Nomenclature
... Cis: both substituents are on the same side of the ring. Trans: substituents are on opposite sides of the ring. ...
... Cis: both substituents are on the same side of the ring. Trans: substituents are on opposite sides of the ring. ...
10. Alkyl Halides
... bromoethane (primary), (c) 2-bromopropane (secondary), and (d) 2-bromo-2methylpropane (tertiary) are successively more hindered, resulting in successively slower SN2 reactions (higher energy Transition States) ...
... bromoethane (primary), (c) 2-bromopropane (secondary), and (d) 2-bromo-2methylpropane (tertiary) are successively more hindered, resulting in successively slower SN2 reactions (higher energy Transition States) ...
Organic Chemistry Notes
... .list the alkyl groups alphabetically .precede each alkyl group by its number .put a dash between each alkyl group and its number Examples Heb p. 220 4-ethyl-2-methyloctane 3-ethyl-3-methyloctane (2 groups can be attached to same carbon) 5-ethyl-3-methyl-6-propyldecane (tricky!!) Rule: If an alkyl g ...
... .list the alkyl groups alphabetically .precede each alkyl group by its number .put a dash between each alkyl group and its number Examples Heb p. 220 4-ethyl-2-methyloctane 3-ethyl-3-methyloctane (2 groups can be attached to same carbon) 5-ethyl-3-methyl-6-propyldecane (tricky!!) Rule: If an alkyl g ...
Worksheets for this unit
... break down these large molecules to useable size is called ___________________. There are two types called ______________ and _________________________. 7. The opposite to cracking is called ____________________________ ...
... break down these large molecules to useable size is called ___________________. There are two types called ______________ and _________________________. 7. The opposite to cracking is called ____________________________ ...
Organic Chemistry Naming Branched Hydrocarbons
... CH3CH3 CH3-CH2-CH2-CH-CH-CH-CH2 –CH3 CH-CH3 CH3 Alkyl groups: 3 methyl groups and 1 ethyl group Alphabetically: ethyl comes before methyl so…. ethyl methyloctane Adding prefixes: none for ethyl, but tri-for the methyl groups so… ethyl trimethyloctane ...
... CH3CH3 CH3-CH2-CH2-CH-CH-CH-CH2 –CH3 CH-CH3 CH3 Alkyl groups: 3 methyl groups and 1 ethyl group Alphabetically: ethyl comes before methyl so…. ethyl methyloctane Adding prefixes: none for ethyl, but tri-for the methyl groups so… ethyl trimethyloctane ...
A mechanistic approach to solvolysis of n-caproyl chloride (n
... reaction which favours S N2 mechanism. With increase in bulkness of alkyl group the magnitude of the enthalpy of activation decreases. Similarly hydrolysis will also have relatively lower magnitude for entropy of activation. This shows that in methanolysis and ethanolysis reaction take places solely ...
... reaction which favours S N2 mechanism. With increase in bulkness of alkyl group the magnitude of the enthalpy of activation decreases. Similarly hydrolysis will also have relatively lower magnitude for entropy of activation. This shows that in methanolysis and ethanolysis reaction take places solely ...
Study Guide Chapter 4 Alcohols and Alkyl Halides
... Propane has six primary hydrogens and two secondary. In the chlorination of propane, the relative proportions of hydrogen atom removal are given by the product of the statistical distribution and the relative rate per hydrogen. Given that a secondary hydrogen is abstracted 3.9 times faster than a pr ...
... Propane has six primary hydrogens and two secondary. In the chlorination of propane, the relative proportions of hydrogen atom removal are given by the product of the statistical distribution and the relative rate per hydrogen. Given that a secondary hydrogen is abstracted 3.9 times faster than a pr ...
Chapter 18 Organic Chemistry - American Public University System
... • Alkenes and alkynes can add hydrogen in hydrogenation reactions. • Hydrogenation reactions convert unsaturated hydrocarbons into saturated hydrocarbons. • Vegetable oil is an unsaturated fat—its carbon chains contain double bonds. Unsaturated fats tend to be liquids at room temperature. • By means ...
... • Alkenes and alkynes can add hydrogen in hydrogenation reactions. • Hydrogenation reactions convert unsaturated hydrocarbons into saturated hydrocarbons. • Vegetable oil is an unsaturated fat—its carbon chains contain double bonds. Unsaturated fats tend to be liquids at room temperature. • By means ...
Propolis (bee glue): an unusual mordant for gilding in Italian
... hive product containing material collected by bees from buds or other plant exudates, volatile substances and beeswax. It is used by bees as a sealant and to protect the nest against microorganisms. Both Aristotle and Pliny give accounts of propolis – mentioning its medicinal properties. Interest in ...
... hive product containing material collected by bees from buds or other plant exudates, volatile substances and beeswax. It is used by bees as a sealant and to protect the nest against microorganisms. Both Aristotle and Pliny give accounts of propolis – mentioning its medicinal properties. Interest in ...
Alcohol

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethyl alcohol (ethanol), the predominant alcohol in alcoholic beverages.The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name. The suffix -ol in non-systematic names (such as paracetamol or cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples glucose and sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.