
CH 106 - Clackamas Community College
... Explain why the molecules of alcohols, aldehydes, and ketones are polar and how this affects their melting points, boiling points, and solubility in water. Perform laboratory tests for alcohols (primary and secondary) and aldehydes. Use infrared spectra to help identify the following types of ...
... Explain why the molecules of alcohols, aldehydes, and ketones are polar and how this affects their melting points, boiling points, and solubility in water. Perform laboratory tests for alcohols (primary and secondary) and aldehydes. Use infrared spectra to help identify the following types of ...
course outline - Clackamas Community College
... Describe how amino acids are joined by peptide bonds to make a protein molecule and know the kinds of reactions involved in making and breaking these bonds. Define and recognize descriptions of the primary, secondary, tertiary and quaternary structure of a protein. Know the four major classes of ...
... Describe how amino acids are joined by peptide bonds to make a protein molecule and know the kinds of reactions involved in making and breaking these bonds. Define and recognize descriptions of the primary, secondary, tertiary and quaternary structure of a protein. Know the four major classes of ...
HILL12_Lecture_09
... that contain a carbon-tocarbon triple bond. Their general formulas are CnH2n-2. Their names begin with a prefix denoting the number of carbon atoms followed by the suffix –yne. Ethyne (acetylene) is the simplest alkyne. © 2010 Pearson Prentice Hall, Inc. ...
... that contain a carbon-tocarbon triple bond. Their general formulas are CnH2n-2. Their names begin with a prefix denoting the number of carbon atoms followed by the suffix –yne. Ethyne (acetylene) is the simplest alkyne. © 2010 Pearson Prentice Hall, Inc. ...
Development of Multi-Component Reactions using Catalytically Generated Allyl Metal Reagents
... to homoallylic alcohols is an important C-C bond forming reaction. Several attractive properties of allylboronates account for their popularity as synthetic intermediates in organic synthesis.8-62 Their high diastereoselectivity in the coupling reactions with aldehydes is predictable and often super ...
... to homoallylic alcohols is an important C-C bond forming reaction. Several attractive properties of allylboronates account for their popularity as synthetic intermediates in organic synthesis.8-62 Their high diastereoselectivity in the coupling reactions with aldehydes is predictable and often super ...
Organic Chemistry
... The Petroleum Industry Crude Oil: Complex mixture of 100’s of hydrocarbon variants →“Cracking”: Separate mixture out by boiling point ...
... The Petroleum Industry Crude Oil: Complex mixture of 100’s of hydrocarbon variants →“Cracking”: Separate mixture out by boiling point ...
An Introduction to Organic Compounds: Nomenclature
... Multivalent atoms are 1º, 2º, or 3º by bonding to C’s. Univalent atom or group not really 1º, 2º, or 3º on its own - ID depends on type of carbon it is bonded to. ...
... Multivalent atoms are 1º, 2º, or 3º by bonding to C’s. Univalent atom or group not really 1º, 2º, or 3º on its own - ID depends on type of carbon it is bonded to. ...
Ch13 Lecture
... D. Addition of Water—Hydration • If the reactant is an asymmetrical alkene, the product will be determined by Markovnikov’s rule. ...
... D. Addition of Water—Hydration • If the reactant is an asymmetrical alkene, the product will be determined by Markovnikov’s rule. ...
Constitutional isomers and stereoisomers
... contains two different functional groups – an alcohol(OH–) group and an alkene group. Draw 3dimensional structures for both enantiomers that clearly show the relationship between them. ...
... contains two different functional groups – an alcohol(OH–) group and an alkene group. Draw 3dimensional structures for both enantiomers that clearly show the relationship between them. ...
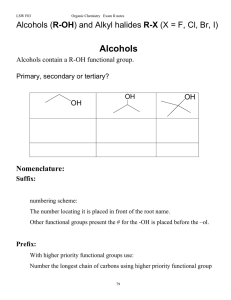
Alcohols (R-OH), and alkyl halides, RX
... Step 2: Which ones give the correct number of products? Step 3: Calculate which has the correct ratios. ...
... Step 2: Which ones give the correct number of products? Step 3: Calculate which has the correct ratios. ...
ation in Cytochrome P-450-Catalyzed Reactions
... whereas distal protonation would give water-complexed ironoxo. In the mutants lacking threonine in the active site, the lifetime (or population if an equilibrium is involved) of the hydroperoxo-iron species is increased. The hydroperoxo-iron species is an effective electrophilic epoxidizing agent, b ...
... whereas distal protonation would give water-complexed ironoxo. In the mutants lacking threonine in the active site, the lifetime (or population if an equilibrium is involved) of the hydroperoxo-iron species is increased. The hydroperoxo-iron species is an effective electrophilic epoxidizing agent, b ...
T_AllylCF3paperBM[5]
... 22). Next, the reaction was optimized for the synthesis of indanes 3a,a' (Table 3). For this purpose alcohol 1a was treated with some acids without benzene as nucleophile. Again the best results were obtained for FeCl3 (Entries 1-2). It is enough to use 0.5 equivalent of FeCl3 to conduct the reactio ...
... 22). Next, the reaction was optimized for the synthesis of indanes 3a,a' (Table 3). For this purpose alcohol 1a was treated with some acids without benzene as nucleophile. Again the best results were obtained for FeCl3 (Entries 1-2). It is enough to use 0.5 equivalent of FeCl3 to conduct the reactio ...
Organic Chemistry Fifth Edition
... • Identify the product isolated when cyclopentanone reacts with dimethyl amine. ...
... • Identify the product isolated when cyclopentanone reacts with dimethyl amine. ...
Sodium tungstate dihydrate - Revista Virtual de Química
... bonds in its structure) into the corresponding epoxy derivatives. This catalytic system possesses much higher epoxidation efficiency than the traditional CH3CO2H/H2O2 system. The proposed method also induces further oxidation of the epoxides into the corresponding ketones and aldehydes, resulting fr ...
... bonds in its structure) into the corresponding epoxy derivatives. This catalytic system possesses much higher epoxidation efficiency than the traditional CH3CO2H/H2O2 system. The proposed method also induces further oxidation of the epoxides into the corresponding ketones and aldehydes, resulting fr ...
Chapter 24. Amines
... • Simple methylated amines from reaction of NH3 with CH3OH and alumina catalyst • Yields a mixture of monomethylated, dimethylated, and trimethylated products that are easily separated by distillation ...
... • Simple methylated amines from reaction of NH3 with CH3OH and alumina catalyst • Yields a mixture of monomethylated, dimethylated, and trimethylated products that are easily separated by distillation ...
Chapter 24. Amines
... • Simple methylated amines from reaction of NH3 with CH3OH and alumina catalyst • Yields a mixture of monomethylated, dimethylated, and trimethylated products that are easily separated by distillation ...
... • Simple methylated amines from reaction of NH3 with CH3OH and alumina catalyst • Yields a mixture of monomethylated, dimethylated, and trimethylated products that are easily separated by distillation ...
Chapter Seventeen
... Unsubstituted amides can form 3 strong hydrogen bonds to other amide molecules. They are higher melting and higher boiling than the acids from which they are derived. Except for the simplest amide (formamide, a liquid), the low molecular-weight unsubstituted amides are solids that are soluble in bot ...
... Unsubstituted amides can form 3 strong hydrogen bonds to other amide molecules. They are higher melting and higher boiling than the acids from which they are derived. Except for the simplest amide (formamide, a liquid), the low molecular-weight unsubstituted amides are solids that are soluble in bot ...
Chapter 11: Sugars and Polysaccharides
... You must be able to recognize the different saccharide derivatives. You are not responsible for learning the names of the derivatives ...
... You must be able to recognize the different saccharide derivatives. You are not responsible for learning the names of the derivatives ...
Alcohol

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethyl alcohol (ethanol), the predominant alcohol in alcoholic beverages.The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name. The suffix -ol in non-systematic names (such as paracetamol or cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples glucose and sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.










![T_AllylCF3paperBM[5]](http://s1.studyres.com/store/data/003584459_1-3decab572f7fca68901a941affab18ea-300x300.png)











