Chapter 2 Phenols
... 5- Reactions of Phenols A hydroxyl group is very powerful activating substituent, and electrophilic aromatic substitution in phenol occurs far faster, and under milder condition, than in benzene. ...
... 5- Reactions of Phenols A hydroxyl group is very powerful activating substituent, and electrophilic aromatic substitution in phenol occurs far faster, and under milder condition, than in benzene. ...
File
... Although they share the same functional group with alcohols, where the –OH group is attached to an aliphatic carbon, the chemistry of phenols is very different from that of alcohols. ...
... Although they share the same functional group with alcohols, where the –OH group is attached to an aliphatic carbon, the chemistry of phenols is very different from that of alcohols. ...
Chapter 12
... of butane in the left-hand structure shown above should rightly be butane because there are four carbon atoms in its backbone. The isomer depicted in the right-hand structure has only a threecarbon backbone, and so it has the base name propane. ...
... of butane in the left-hand structure shown above should rightly be butane because there are four carbon atoms in its backbone. The isomer depicted in the right-hand structure has only a threecarbon backbone, and so it has the base name propane. ...
Aldehydes and Ketones
... Naming Aldehydes IUPAC Replace the -e in the alkane name with –al Common Add aldehyde to the prefixes form (1C), acet (2C), propion(3), and butry(4C) O ...
... Naming Aldehydes IUPAC Replace the -e in the alkane name with –al Common Add aldehyde to the prefixes form (1C), acet (2C), propion(3), and butry(4C) O ...
Document
... Counting from each direction find the carbon where the alkyl group is attached (the lower number) J Deutsch 2003 ...
... Counting from each direction find the carbon where the alkyl group is attached (the lower number) J Deutsch 2003 ...
Learning Guide for Chapter 16
... Are ethers highly reactive, or highly unreactive functional groups? What kind of reagents do ethers react with? strong acids H-base ...
... Are ethers highly reactive, or highly unreactive functional groups? What kind of reagents do ethers react with? strong acids H-base ...
Electrophilic Additions: Alkenes Addition of Hydrogen Halides
... In a regioselective reaction, one constitutional isomer is the major or the only product. I: early transition state (Like reactants) ...
... In a regioselective reaction, one constitutional isomer is the major or the only product. I: early transition state (Like reactants) ...
Ch04-04-alkenes-2
... Exergonic reaction: early transition state resembles reactants (I). Endergonic reaction: late transition state resembles products (II). ...
... Exergonic reaction: early transition state resembles reactants (I). Endergonic reaction: late transition state resembles products (II). ...
Carboxylic acids and derivatives
... Electron-withdrawing groups (e.g NO2) enhance the acid strength while electron-donating groups ( e.g alkyl ) decrease the acid strength. Effects are strongest for substituents in the ortho and para ...
... Electron-withdrawing groups (e.g NO2) enhance the acid strength while electron-donating groups ( e.g alkyl ) decrease the acid strength. Effects are strongest for substituents in the ortho and para ...
Addition Reactions of Carbonyls Part 1
... electrophile is added (leaving neutral acetone molecules as the only available electrophiles) O ...
... electrophile is added (leaving neutral acetone molecules as the only available electrophiles) O ...
Lectures 34-35
... The reason that this reaction is favoured in the forward direction is that the products are more stable than the reactants. In particular, the conjugate base (acetate; CH3CO2-) is much more stable than the original base (hydroxide, OH-). This makes hydroxide a stronger base than acetate. It also mak ...
... The reason that this reaction is favoured in the forward direction is that the products are more stable than the reactants. In particular, the conjugate base (acetate; CH3CO2-) is much more stable than the original base (hydroxide, OH-). This makes hydroxide a stronger base than acetate. It also mak ...
Stereoselective reactions of the carbonyl group
... • These can result in fairly spectacular allylation reactions (higher ee than this example) • A problem frequently arises with crotylation • Often the reactions proceed with poor diastereoselectivity favouring either the syn ...
... • These can result in fairly spectacular allylation reactions (higher ee than this example) • A problem frequently arises with crotylation • Often the reactions proceed with poor diastereoselectivity favouring either the syn ...
Lectures 37-38 - U of L Class Index
... The reason that this reaction is favoured in the forward direction is that the products are more stable than the reactants. In particular, the conjugate base (acetate; CH3CO2-) is much more stable than the original base (hydroxide, OH-). This makes hydroxide a stronger base than acetate. It also mak ...
... The reason that this reaction is favoured in the forward direction is that the products are more stable than the reactants. In particular, the conjugate base (acetate; CH3CO2-) is much more stable than the original base (hydroxide, OH-). This makes hydroxide a stronger base than acetate. It also mak ...
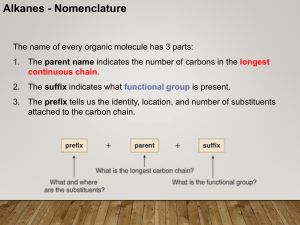
CHEM 210 Nomenclature Lecture 1
... Some organic compounds are identified using common names that do not follow the IUPAC system of nomenclature. Many of these names were given long ago before the IUPAC system was adopted, and are still widely used. Additionally, some names are descriptive of shape and structure, like those below: ...
... Some organic compounds are identified using common names that do not follow the IUPAC system of nomenclature. Many of these names were given long ago before the IUPAC system was adopted, and are still widely used. Additionally, some names are descriptive of shape and structure, like those below: ...
Lectures 37-38 - U of L Class Index
... The reason that this reaction is favoured in the forward direction is that the products are more stable than the reactants. In particular, the conjugate base (acetate; CH3CO2-) is much more stable than the original base (hydroxide, OH-). This makes hydroxide a stronger base than acetate. It also mak ...
... The reason that this reaction is favoured in the forward direction is that the products are more stable than the reactants. In particular, the conjugate base (acetate; CH3CO2-) is much more stable than the original base (hydroxide, OH-). This makes hydroxide a stronger base than acetate. It also mak ...
FSII ch09 presentation_modified
... testing employees for drug use, screening athletes for drug use, and testing sexual assault victims for the presence of date rape drugs. ...
... testing employees for drug use, screening athletes for drug use, and testing sexual assault victims for the presence of date rape drugs. ...
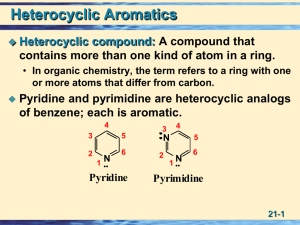
Organic Chemistry - City University of New York
... cyclopentadienyl anion occupy the p1, p2, and p3 molecular orbitals, all of which are bonding. ...
... cyclopentadienyl anion occupy the p1, p2, and p3 molecular orbitals, all of which are bonding. ...
Alcohol

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethyl alcohol (ethanol), the predominant alcohol in alcoholic beverages.The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name. The suffix -ol in non-systematic names (such as paracetamol or cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples glucose and sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.