* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 2 Phenols
Homoaromaticity wikipedia , lookup
Aromaticity wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Ene reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Aromatization wikipedia , lookup
Asymmetric induction wikipedia , lookup
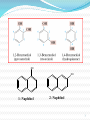
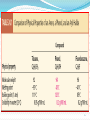
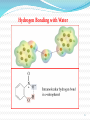
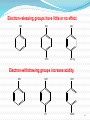
1 1-Nomenclature of Phenols Phenols are compounds that have hydroxyl group bonded directly to benzene ring. Named on basis of phenol as parent, substituents listed in alphabetical order. 2 OH OH 1- Naphthol 2- Naphthol 3 4 2-Physical Properties 1-The OH group of phenols allows hydrogen bonding to other phenol molecules and to water. 2-Compared to compounds of similar size and molecular weight, hydrogen bonding in phenol raises its melting point, boiling point, and solubility in water. 5 6 Hydrogen Bonding of Phenols O H 24 - 7 O Hydrogen Bonding with Water 8 3- Acidity of Phenols Most characteristic property of phenols is their acidity. Phenols(pKa = 10) are more acidic than alcohols(pKa = 1620) but less acidic than Carboxylic acids (pKa = 5). 9 To explain why, let’s compare the ionization of Ethanol and Phenols 10 11 12 Substituent Effects on the Acidity of Phenols. 1-Electron-releasing groups ( CH3, OCH3) have little or no effect. 2-Electron-withdrawing groups ( NO2, Cl, OH) increase acidity. 3- Multiple substituent of electron- withdrawing group greatly increase acidity of phenols. 13 Electron-releasing groups have little or no effect. OH OH OH CH3 OCH3 Electron-withdrawing groups increase acidity. OH OH OH Cl NO2 14 Effect of electron-withdrawing groups is most pronounced at ortho and para positions. OH OH OH NO2 NO2 NO2 Effect of strong electron-withdrawing groups is cumulative. OH OH OH NO2 NO2 NO2 O2 N NO2 NO2 15 16 4-Sources of Phenols Phenols was first isolated from coal tar. Phenol is an important industrial chemical. Major use is in phenolic resins for adhesives and plastics. 17 Industrial preparation of Phenols 18 Laboratory Synthesis of Phenols 19 5- Reactions of Phenols A hydroxyl group is very powerful activating substituent, and electrophilic aromatic substitution in phenol occurs far faster, and under milder condition, than in benzene. a- Halogenation 20 b- Sulfonation 21 c- Nitration d- Nitrosation 22 d-Friedal Crafts Alkylation e-Friedal Crafts Acylation 23 In the absence of ALCL3, However Oacylation occurs instead. 24 25 f- Reaction with arenediazonium salts. 26 6- Aspirin and the Klobe – Schmitt Reaction. 27 Preparation of Salicylic Acid This reaction is called the Kolbe- Schmitt reaction. Acidification coverts the sodium salt shown above to salicylic acid. 28