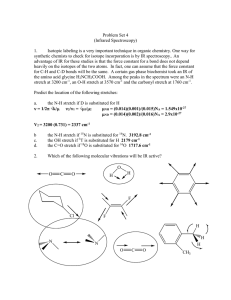
PHYSICAL SETTING CHEMISTRY
... (1) lose electrons and have a decrease in oxidation number (2) lose electrons and have an increase in oxidation number (3) gain electrons and have a decrease in oxidation number (4) gain electrons and have an increase in oxidation number ...
... (1) lose electrons and have a decrease in oxidation number (2) lose electrons and have an increase in oxidation number (3) gain electrons and have a decrease in oxidation number (4) gain electrons and have an increase in oxidation number ...
Chemistry for Changing Times 11th Edition Hill and Kolb
... that contain a carbon-tocarbon triple bond. Their general formulas are CnH2n-2. Their names begin with a prefix denoting the number of carbon atoms followed by the suffix –yne. Ethyne (acetylene) is the simplest alkyne. ...
... that contain a carbon-tocarbon triple bond. Their general formulas are CnH2n-2. Their names begin with a prefix denoting the number of carbon atoms followed by the suffix –yne. Ethyne (acetylene) is the simplest alkyne. ...
chemistry 1000
... Nomenclature also makes it easier for us to talk about organic chemistry in situations where we can’t just draw the molecules. The rules for organic nomenclature are the same as those you learned in CHEM 2000 (see next page); however, we will now deal with a few more functional groups, a few more di ...
... Nomenclature also makes it easier for us to talk about organic chemistry in situations where we can’t just draw the molecules. The rules for organic nomenclature are the same as those you learned in CHEM 2000 (see next page); however, we will now deal with a few more functional groups, a few more di ...
Chapter 11: macromolecules
... This chapter is devoted to the study of polymers, huge molecules composed of tens to thousands of repeating monomer units. Nature makes a wide variety of polymers. For example, DNA, starch, fibre, enzymes, hair, skin, fingernails, cotton, wool, and silk, are all naturally-occurring polymers. Some ex ...
... This chapter is devoted to the study of polymers, huge molecules composed of tens to thousands of repeating monomer units. Nature makes a wide variety of polymers. For example, DNA, starch, fibre, enzymes, hair, skin, fingernails, cotton, wool, and silk, are all naturally-occurring polymers. Some ex ...
CH 2
... that contains one or more carbon – carbon double bonds. ALKYNES – An unsaturated hydrocarbon that contains one or more carbon – carbon triple bonds. AROMATIC – Organic compounds that contains the characteristics of benzene & benzene ring in its structure. ...
... that contains one or more carbon – carbon double bonds. ALKYNES – An unsaturated hydrocarbon that contains one or more carbon – carbon triple bonds. AROMATIC – Organic compounds that contains the characteristics of benzene & benzene ring in its structure. ...
Course Structure - Indian Association for the Cultivation of Science
... Applications to one-, two- and three-dimensional systems, degeneracy, Jahn-Teller effect. LHO(in terms of ladder/creation-annihilation operators), rigid rotor(including electron in a ring), hydrogen atom(ladder operators). One-dimensional periodic potential; Bloch theorem, band structure. Angular mo ...
... Applications to one-, two- and three-dimensional systems, degeneracy, Jahn-Teller effect. LHO(in terms of ladder/creation-annihilation operators), rigid rotor(including electron in a ring), hydrogen atom(ladder operators). One-dimensional periodic potential; Bloch theorem, band structure. Angular mo ...
21. Characterization of the Organic Matter in a Site 147 Core from
... to deuterated alcohols by lithium aluminum deuteride. The alcohols were converted to iodides and then further reduced to deuterated hydrocarbons by lithium aluminum deuteride. The steps are outlined using a carboxylic acid as an example. RCOOH + LiAlD4 - RCD2OH RCD2OH + HI ^ ...
... to deuterated alcohols by lithium aluminum deuteride. The alcohols were converted to iodides and then further reduced to deuterated hydrocarbons by lithium aluminum deuteride. The steps are outlined using a carboxylic acid as an example. RCOOH + LiAlD4 - RCD2OH RCD2OH + HI ^ ...
Nomenclature of Organic Compounds
... b. Two substituents, cite them alphabetically and give the number 1 position to the first substituent. c. Three or more substituents, the substituent given the number 1 is the one that results in a second substituent getting as low a number as possible. ...
... b. Two substituents, cite them alphabetically and give the number 1 position to the first substituent. c. Three or more substituents, the substituent given the number 1 is the one that results in a second substituent getting as low a number as possible. ...
msc final chemistry - Dr. CV Raman University
... Dr. C.V. Raman University Kargi Road Bilaspur (C.G.) DEPARTMENT OF CHEMISTRY LIST OF EXPERIMENT (M.SC.) Analytical Practical (Sem. - II) ...
... Dr. C.V. Raman University Kargi Road Bilaspur (C.G.) DEPARTMENT OF CHEMISTRY LIST OF EXPERIMENT (M.SC.) Analytical Practical (Sem. - II) ...
- ILM.COM.PK
... Recall that polyesters and polyamides are formed by condensation polymerisation Recall the properties and uses of some condensation polymers Work out the repeating unit in condensation polymers Recall that peptides are examples of condensation polymers ...
... Recall that polyesters and polyamides are formed by condensation polymerisation Recall the properties and uses of some condensation polymers Work out the repeating unit in condensation polymers Recall that peptides are examples of condensation polymers ...
03 Inorg. drugs with acid-base prop. IOC of С,Al, Ba,Ag
... Reaction with hydrochloric acid. Dissolve about 10 mg of the substance to be examined in 10 ml of water R or use 10 ml of the prescribed solution. Add 0.3 ml of hydrochloric acid R1. A curdled, white precipitate is formed that dissolves on addition of 3 ml of dilute ammonia R1. AgNO3 + HCl = AgCl + ...
... Reaction with hydrochloric acid. Dissolve about 10 mg of the substance to be examined in 10 ml of water R or use 10 ml of the prescribed solution. Add 0.3 ml of hydrochloric acid R1. A curdled, white precipitate is formed that dissolves on addition of 3 ml of dilute ammonia R1. AgNO3 + HCl = AgCl + ...
Chapter 4 Stereochemistry and Chirality Flow chart for determining
... • This inversion process, which is an equilibrium, will take a single chiral isomer into a racemic mixture. Any process that allows one chiral isomer to interconvert with its enantiomer is termed racemization. • Since amine inversion can be fast at room temperature, it is often impossible to isolate ...
... • This inversion process, which is an equilibrium, will take a single chiral isomer into a racemic mixture. Any process that allows one chiral isomer to interconvert with its enantiomer is termed racemization. • Since amine inversion can be fast at room temperature, it is often impossible to isolate ...
Organometallics II
... formula C11H22O, are formed in the reaction of methyl lithium with 3-(R)-tertbutylcyclohexanone. These two alcohols are ...
... formula C11H22O, are formed in the reaction of methyl lithium with 3-(R)-tertbutylcyclohexanone. These two alcohols are ...
organonitrogen compounds i. amines
... moment than 13C, it is very difficult to detect I5N resonances at the naturalabundance level.2 Indeed, natural 15N has to be observed for about a 6 x 101° longer time than protons to achieve the same signal-to-noise ratio! Despite this difficulty, natural-abundance 15N spectra can be obtained for ma ...
... moment than 13C, it is very difficult to detect I5N resonances at the naturalabundance level.2 Indeed, natural 15N has to be observed for about a 6 x 101° longer time than protons to achieve the same signal-to-noise ratio! Despite this difficulty, natural-abundance 15N spectra can be obtained for ma ...
Reactions of Alcohols
... The ZnCl2 coordinates to the hydroxyl oxygen, and this generates a far superior leaving group. Primary alcohols react in a similar fashion except the free cation is not generated, and the substitution is of S N2 ...
... The ZnCl2 coordinates to the hydroxyl oxygen, and this generates a far superior leaving group. Primary alcohols react in a similar fashion except the free cation is not generated, and the substitution is of S N2 ...
File - need help with revision notes?
... Manufacture of TNT (Trinitrotoluene) Methyl benzene reacts with nitric acid to form 2,4,6 trinitrotoluene and water. Halogenation of Benzene Electrophilic substitution with a halogen in the presence of a halogen carrier catalyst (FeBr3 or AlCl3) Benzene is unable to react with a halogen alone bec ...
... Manufacture of TNT (Trinitrotoluene) Methyl benzene reacts with nitric acid to form 2,4,6 trinitrotoluene and water. Halogenation of Benzene Electrophilic substitution with a halogen in the presence of a halogen carrier catalyst (FeBr3 or AlCl3) Benzene is unable to react with a halogen alone bec ...
Completed Notes for Organic Chemistry
... • Structural isomers are those which arise from the differing bonding connections among atoms in a molecule. • Stereo isomers are isomers in which the bonding connections remain unchanged, but the spatial arrangements of specific groups relative to one another differ. • One type of stereoisomerism i ...
... • Structural isomers are those which arise from the differing bonding connections among atoms in a molecule. • Stereo isomers are isomers in which the bonding connections remain unchanged, but the spatial arrangements of specific groups relative to one another differ. • One type of stereoisomerism i ...
Mechanism of Action of Sodium Hypochlorite - FORP
... and fat solvent degrading fatty acids, transforming them into fatty acid salts (soap) and glycerol (alcohol), that reduces the surface tension of the remaining solution (scheme 1 - saponification reaction). Sodium hypochlorite neutralizes amino acids forming water and salt (scheme 2 - neutralization ...
... and fat solvent degrading fatty acids, transforming them into fatty acid salts (soap) and glycerol (alcohol), that reduces the surface tension of the remaining solution (scheme 1 - saponification reaction). Sodium hypochlorite neutralizes amino acids forming water and salt (scheme 2 - neutralization ...
Sec 5.8 - 5.11 notes
... 9) Electrons do not travel through the ______ _______, only through the _______. 10) Ions (cations & anions) do not travel through the wire but only through the _____________. 11) The salt bridge can contain any ______________. 12) The anode will ________(gains/loses) mass as it is __________(oxidiz ...
... 9) Electrons do not travel through the ______ _______, only through the _______. 10) Ions (cations & anions) do not travel through the wire but only through the _____________. 11) The salt bridge can contain any ______________. 12) The anode will ________(gains/loses) mass as it is __________(oxidiz ...
Aromatic amines The
... A Nitrogen, containing a lone pair is the key atom Resembles Ammonia where one or more H’s Are replaced by alkyl groups The lone pair participates in the reactivity Of amines Amines are a core part of ‘amino acids’ ...
... A Nitrogen, containing a lone pair is the key atom Resembles Ammonia where one or more H’s Are replaced by alkyl groups The lone pair participates in the reactivity Of amines Amines are a core part of ‘amino acids’ ...
View/Open - AURA - Alfred University
... mediate the Ritter reaction under mild conditions and it may now be possible to use unprotected, acid-sensitive functional groups. In an attempt to determine the scope of the PEDOT-mediated Ritter reaction, a number of alcohols were reacted with acetonitrile. Given that these reactions used acetonit ...
... mediate the Ritter reaction under mild conditions and it may now be possible to use unprotected, acid-sensitive functional groups. In an attempt to determine the scope of the PEDOT-mediated Ritter reaction, a number of alcohols were reacted with acetonitrile. Given that these reactions used acetonit ...
Downloaded from www.studiestoday.com Downloaded from www
... sequence. This sequence of amino acids is said to be the primary structure of proteins. Secondary structure of proteins: The secondary structure of proteins refers to the shape in which a long polypeptide can exist. The two different secondary structure possible are α -Helix structure and β – pleate ...
... sequence. This sequence of amino acids is said to be the primary structure of proteins. Secondary structure of proteins: The secondary structure of proteins refers to the shape in which a long polypeptide can exist. The two different secondary structure possible are α -Helix structure and β – pleate ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.