* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Nomenclature of Organic Compounds
Survey
Document related concepts
Transcript
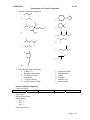
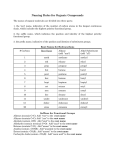
CHEM 109A CLAS Nomenclature of Organic Compounds 1. Name the following compounds. a. e. f. OH Br b. OH g. OH h. NH 2 c. Cl i. NH2 j. d. 2. Draw the following compounds. a. 3-chloro-2,3dimethylcyclopentanol b. 4,5-diethyl-3-iodo-4octanamine c. sec-butyl chloride d. tert-butyl isobutyl ether Steps for naming compounds 5 parts to a name… Stereoisomerism Substituents Parent Hydrocarbon Stereoisomerism – Substituents – Parent Hydrocarbon – Unsaturation – -ane → -ene → -yne → Functional Group – e. f. g. h. i. j. neopentyl alcohol dimethyl ether ethene methanal ethanoic acid isobutyl amine Unsaturation Functional Group Page 1 of 4 CHEM 109A CLAS Nomenclature of Organic Compounds Alkanes (CnH2n+2): , Alkenes (CnH2n): , Alkynes (CnH2n-2): 1. Determine the # of C atoms in the longest continuous chain (parent chain) – Watch for branches! # of carbons Stem Name of alkane Molecular formula of alkane 1 meth2 eth3 prop4 but5 pent6 hex7 hept8 oct9 non10 dec11 undec12 dodec2. Number the chain in the direction that gives any substituent a lower number. (Numbers are not used in common names) a. If numbering in both directions gives the same number to a substituent, number in the direction that gives the lowest number to the alphabetically first substituent. 3. Substituents are listed in alphabetical order. a. Numbers and words are separated by hyphens and numbers are separated by commas. b. Prefixes are used when there is more than one of the same substituent i. di, tri, tetra, sec and tert are ignored in alphabetizing. ii. iso, neo and cyclo (INC) are NOT ignored in alphabetizing. 4. If there are two chains with the same number of Cs, choose the one with the most substituents as the parent chain. EX. 2,3-dimethylhexane Cycloalkanes: 1. Ring is parent unless the substituent has more Cs than the ring 2. If the ring has a. Only one substituent do NOT number it b. Two substituents, cite them alphabetically and give the number 1 position to the first substituent. c. Three or more substituents, the substituent given the number 1 is the one that results in a second substituent getting as low a number as possible. Page 2 of 4 CHEM 109A CLAS Nomenclature of Organic Compounds i. If two substituents have the same low number, the ring is numbered in a direction that gives the third substituent the lowest possible number. 1,1-dimethylcyclohexane EX. Alkyl Halides: 1. Name of the alkyl group followed by the name of the halogen with –ide ending. 2. Alkyl halides are named as substituent alkanes. The prefixes for the halogen end with “o” (fluoro, chloro, bromo, iodo). Cl EX. 2-fluoropropane Table 2.2 Names of Common Alkyl Groups Name Chemical Formula Methyl Ethyl Propyl Isopropyl Butyl Isobutyl sec-butyl tert-butyl Pentyl Isopentyl Hexyl Isohexyl Page 3 of 4 CHEM 109A CLAS Nomenclature of Organic Compounds Ethers: Common Name: 1. Name the two alkyl substituents in alphabetical order, followed by the word “ether”. Systematic Name: 2. Name the ether as an alkane with an RO substituent (replace the –yl ending with – oxy). EX. ethoxy ethane O Alcohols: Common Name: 1. Name the alkyl group to which the OH group is attached, followed by the word “alcohol”. Systematic Name: 2. Replace the –e ending of the parent hydrocarbon with the suffix –ol. Cl EX. 5-methyl-3-hexanol HO Amines: Common Name: 1. Name the alkyl groups bonded to the nitrogen in alphabetical order, followed by the word “amine” – the entire name is written as one word. Systematic Name: 2. A number denotes the carbon to which the nitrogen is attached (can appear before the name, or before the “amine”). 3. Replace the –e ending of the parent hydrocarbon with the word “amine”. 4. Substituents – whether attached to the nitrogen or to the parent hydrocarbon – are listed in alphabetical order, and a number or and “N” is assigned to each one. EX. N N,N-dimethyl-3-pentamine Page 4 of 4