* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 2: Nomenclature and Structure
Survey
Document related concepts
Transcript
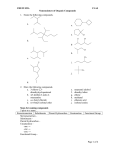
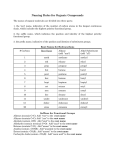
Chemistry 241 Chapter 2: An Intro to Organic Compounds Homework: 45 a-j, o, 46, 48, 51 a-d, 54 a-i, 55, 58, 60, 61, 62 a-c, f, 66, 69 a-c, 73 I. Terms A. Generic 1. 2. 3. 4. alkane: all single bonds hydrocarbon: carbon and hydrogen substituent: something hanging off of chain; not part of chain straight chain vs branched chain 5. homologous series: add a carbon to chain to continue series a. methylene –(CH2)6. isomer a. constitutional isomers: same formula, different connectivity i. butane vs isobutene, 1-chloro vs 2-chloropropane ii. isomers of C4H10 (2), C5H12 (3), C6H14 (5) b. stereo isomers (chapter 3 and later) 7. conformers a. same molecule drawn from different perspective B. Nomenclature 1. systematic (IUPAC) vs common a. every name is unique to one molecule b. IUPAC gives one name to every molecule, though it may have several common names c. IUPAC has prefix-stem (or root)-suffix i. prefixes are substituent(s) (may be more than one) ii. root/stem is parent molecule iii. suffix functional group of parent (alkane, alcohol, etc) d. Common names almost always are without numbers 2. alkanes a. longer table on p 72 # C’s Parent name Substituent name 1 methane methyl 2 ethane ethyl 3 propane propyl 4 butane butyl 5 pentane pentyl 6 hexane hexyl 7 heptane heptyl 1 8 9 10 octane nonane decane octyl nonyl decyl 3. drawings (for butane) a. condensed CH3CH2CH2CH3 Structure Functional grp alcohol amine Alkyl halide ether b. line diagrams (skeletal structures) 4. branched Common name IUPAC name isobutene 2-methylpropane isopentane 2-methylbutane isohexane 2-methylpentane 5. functional groups (IUPAC) Structure Prefix -OH hydroxy-NH2 amino-F fluoro-Cl chloro-Br bromo-I iodoC-O-C methoxyethoxyetc Suffix -ol -amine C. miscellaneous 1. Types of carbons, hydrogens, amines a. primary (1º) i. C, N connected to one other C ii. H attached to C which is connected to one other C b. secondary (2º) i. C, N connected to two other C’s ii. H attached to C which is connected to two other C’s c. tertiary (3º) i. C, N connected to three other C’s ii. H attached to C which is connected to three other C 2 d. quaternary (4º) i. C, N connected to four other C ii. H not possible 2. more alkyl substituents R a. butyl-, n-butylb. isobutyl-, (IUPAC = (2-methylpropyl)) R R c. sec-butyl R II. d. tert-butyl Alkane Nomenclature A. IUPAC system 1. prefix – stem (or root) - suffix B. Straight and branched chains p78 1. find longest carbon chain a. longest carbon chain with parent (chain includes alcohol, for ex) 2. if a tie, parent chain (two or more possible), parent is one that has more substituents (more substituents = easier to name) 3. name and number substituents a. substituent is prefix b. number from side that gives smaller number c. common names almost never have number 4. if more than one substituent a. if more than one of same substituent, use di-, tri-, tetra-, penta-, hexa-, heptai. 1,1 dichloroethane b. for every substituent, there is a number c. substituents are put into alphabetical order i. di, tri, tetra, (etc), sec, and tert ignored for alphabetizing ii. iso and cyclo count for alphabetizing iii. cyclopentyl before isobutyl, dibromo before chloro 5. numbering more than one substituent a. number from side that gives lowest first substituent number b. if tie, keep going until tie-breaker reached (1st point of difference) 3 C. D. E. F. G. 6. can use “iso-,” “sec-,” or “tert-,” but IUPAC substituent on a substituent method preferred 7. moosh entire thing into one word a. dashes separate words and numbers b. commas separate numbers Cyclics p82 1. count the carbons and add “cyclo” in front 2. if one substituent, no need for numbers 3. if alkyl substituents, either can be parent 4. if more than one substituent, give both numbers, 1st in alphabet is number 1 Alkyl halides p85 1. common names a. no numbers b. sec, iso indicate location of halogen 2. IUPAC already covered Ethers p86 1. common a. carbon chains on either side are substiuents b. two words c. parent and suffix is ether d. sec, iso indicate location of oxygen e. ethyl isobutyl ether, dimethyl ether 2. IUPAC a. smaller, less complicated carbon chain and oxygen are substituent b. name change of substituent: drop “yl” and “oxy” i. CH3- methyl becomes CH3O- methoxy Alcohols p87 1. common a. carbon chain is substituent, parent and suffix = alcohol b. two words c. sec, iso indicate location of halogen d. methyl alcohol, isopropyl alcohol 2. IUPAC a. longest carbon chain containing the –OH functional grp b. suffix is “ol” instead of “ane” c. straight chains: number from side that gives lowest number to -OH d. where to put number i. can put it immediately before “ol” ii. or immediately before root iii. 2-butanol or butan-2-ol e. cyclics: alcohol is number 1, no need to give number if more than one substituent Amines p89 4 1. a. 2. a. b. III. IV. common name all alkyl substituents in front, parent and suffix = amine IUPAC if primary, can name amine as “amino” substituents or, if primary, can name amine as parent i. drop “e” from “ane,” and add amine c. if 2º, 3º, additional chains, alkyl substituents on nitrogen given “N” designation d. alphabetize substituents (N treated as number, not for alphabet) e. if 4º, = quaternary ammonium salt i. name as salt (cation, then anion) ii. list four alkyl groups, then ammonium H. Summary p91 Physical Properties A. Boiling 1. What is it/ what happens 2. Intermolecular forces a. H-bonding b. dipole-dipole attractions c. induced dipole- induced dipole, London dispersion, Van der Waals forces 3. Tables of physical properties of different functional grps in Appendix I B. Melting/Freezing 1. crystal packing C. Solubility 1. like dissolves like 2. polar solvents like polar molecules 3. non-polar solvents like non-polar solutes 4. if immiscible, less dense floats sp3 carbon bonds A. Properties 1. 109.5º 2. free rotation, unlike sp2 or sp B. Newman projections 1. sawhorse projections 2. ethane a. eclipsed b. staggered (conformers) c. 60º clicks 3. butane a. looking down C2-C3 bond b. anti c. gauche d. steric strain 4. energy diagrams p102, and p104 5 C. Cyclohexane 1. chair a. my drawing b. book c. Newman 2. axial and equatorial a. axial b. equitorial 3. ring flip a. what was axial is now equatorial and verse-visa 4. boat 5. strains (p109 table of energies) a. chair lower in energy than boat b. cyclic strain of propane, butane 6. energy diagrams p110 7. axial groups interact a. larger groups prefer equatorial so no interference b. ring flip to get there 8. disubstituted cyclohexanes a. 1,2 or 1,3 or 1,4 b. cis c. trans d. energy of cis, trans, ringflips Objectives Knowledge Memorize the terms for the first ten alkanes (methane decane) (p72) Recall how to name alcohols (drop “-e” on alkane, add “-ol”) and amines Remember the common names isopropyl, isobutyl, tertiary (tert-, or t-) butyl, n- (normal) Recall the rules for IUPAC naming of alcohols, amines, alkyl halides, ethers, and alkanes (including cyclic compounds) Define the terms anti and gauche, eclipsed and staggered, boat and chair, axial and equatorial, cis and trans, Newman and sawhorse projections Comprehension Explain how inter- and intramolecular forces affect physical properties (mp, solubility..) Understand the classification system for carbons, hydrogens, and amines (1°, 2°, 3°, 4° ) Recognize the contributions to the stability of ring size, anti and gauche, eclipsed and staggered, boat and chair, axial and equatorial, cis and trans, Application Use IUPAC rules to name Lewis structures and line diagrams From the IUPAC name, be able to draw the structure Employ energy diagrams to show relative energy of conformers (eclipsed/staggered, etc.) 6