Microsoft Word - Final Exam Study Guide
... A. How many hydrogens? How many lone pairs? B. Label the structure with any missing formal charges. C. Draw at least two more resonance structures and rank them from most major contributor to most minor contributor. D. Mark all the chiral centers and label any designated chiral centers R or S. B. Wo ...
... A. How many hydrogens? How many lone pairs? B. Label the structure with any missing formal charges. C. Draw at least two more resonance structures and rank them from most major contributor to most minor contributor. D. Mark all the chiral centers and label any designated chiral centers R or S. B. Wo ...
sn2 reactions of alkyl halides
... The alkyl halides play an important role in organic synthesis. They can be easily prepared from alcohols or alkenes, among other starting materials. They in turn can be used in the synthesis of a large number of functional groups. These syntheses are often carried out by nucleophilic substitution re ...
... The alkyl halides play an important role in organic synthesis. They can be easily prepared from alcohols or alkenes, among other starting materials. They in turn can be used in the synthesis of a large number of functional groups. These syntheses are often carried out by nucleophilic substitution re ...
Discussion Worksheet #10 Formation of Alcohols Skill 1: Functional
... There are ways to control regiochemistry and stereochemistry with alcohol formation ...
... There are ways to control regiochemistry and stereochemistry with alcohol formation ...
1-Ethyl-4-hydroxy-2-oxo-1,2-dihydroquinoline-3
... A possibility of N,N'-carbonyldiimidazole use in the synthesis of 3', 4'diflurorineanilides of 1-R-2-oxo-4-hydroxyquinolie-3-carboxylic acids has been studied. Their structure is confirmed by NMR spectroscopy and counter synthesis. The results of the antituberculosis activity of the compounds obtain ...
... A possibility of N,N'-carbonyldiimidazole use in the synthesis of 3', 4'diflurorineanilides of 1-R-2-oxo-4-hydroxyquinolie-3-carboxylic acids has been studied. Their structure is confirmed by NMR spectroscopy and counter synthesis. The results of the antituberculosis activity of the compounds obtain ...
+ H 2 SO 4(aq) - Rothschild Science
... Reactivity of a metal makes a difference! If a metal is more reactive than the metal it is displacing a rxn will occur. If the metal is less reactive than the metal it is displacing, a rxn will not occur. ...
... Reactivity of a metal makes a difference! If a metal is more reactive than the metal it is displacing a rxn will occur. If the metal is less reactive than the metal it is displacing, a rxn will not occur. ...
enzyme inhibition
... half died and half didn’t, but I didn’t know. Do you?” Although Sarah had planned to side-track the professor, her story played nicely into his lesson plan. After all, the incident was related to the current class topic of enzymes and enzyme inhibition. The clever professor began, “Let me remind all ...
... half died and half didn’t, but I didn’t know. Do you?” Although Sarah had planned to side-track the professor, her story played nicely into his lesson plan. After all, the incident was related to the current class topic of enzymes and enzyme inhibition. The clever professor began, “Let me remind all ...
exam review - hrsbstaff.ednet.ns.ca
... 17. Calculate the equilibrium constant Kc at 25 oC for the reaction 2 NOCl(g) ↔ 2 NO(g) + Cl2(g) using the following information. In one experiment 2.00 mol of NOCl is placed in a 1.00 -L flask, and the concentration of NO after equilibrium is achieved is 0.66 mol/L. 18. For the gas phase reaction H ...
... 17. Calculate the equilibrium constant Kc at 25 oC for the reaction 2 NOCl(g) ↔ 2 NO(g) + Cl2(g) using the following information. In one experiment 2.00 mol of NOCl is placed in a 1.00 -L flask, and the concentration of NO after equilibrium is achieved is 0.66 mol/L. 18. For the gas phase reaction H ...
Topic 20 Organic Chemistry
... Identify the feature which both molecules possess that accounts for this property. When 2-hydroxypropanoic acid is formed from 2-chloropropanoic acid, the product shows no optical activity. Deduce the type of nucleophilic substitution that takes place and explain your answer. ...
... Identify the feature which both molecules possess that accounts for this property. When 2-hydroxypropanoic acid is formed from 2-chloropropanoic acid, the product shows no optical activity. Deduce the type of nucleophilic substitution that takes place and explain your answer. ...
Chapter 21 Worksheet
... that gives the lowest possible positions. If both directions give the same numbers, the double bond will take priority. c. If there are no functional groups, give the substituents the lowest possible numbers. If you get the same numbers from either end assign the alphabetically first substituent the ...
... that gives the lowest possible positions. If both directions give the same numbers, the double bond will take priority. c. If there are no functional groups, give the substituents the lowest possible numbers. If you get the same numbers from either end assign the alphabetically first substituent the ...
Chapter 10
... This is much smaller than a covalent bond (O-H ~ 104 Kcal/mol) but a compound can have multiple hydrogen bonds that need to be broken to “escape” the liquid phase ...
... This is much smaller than a covalent bond (O-H ~ 104 Kcal/mol) but a compound can have multiple hydrogen bonds that need to be broken to “escape” the liquid phase ...
Review Package
... 11) Write the chemical formulas for the following compounds: a) Sodium iodide b) Carbon monoxide c) Nitrogen triiodide d) Hydrochloric acid e) Sodium phosphate f) Nitrogen gas g) Magnesium oxide h) Phosphoric acid i) Potassium chlorate j) Beryllium nitrate k) Sulphuric acid l) Aluminum sulphide m)Hy ...
... 11) Write the chemical formulas for the following compounds: a) Sodium iodide b) Carbon monoxide c) Nitrogen triiodide d) Hydrochloric acid e) Sodium phosphate f) Nitrogen gas g) Magnesium oxide h) Phosphoric acid i) Potassium chlorate j) Beryllium nitrate k) Sulphuric acid l) Aluminum sulphide m)Hy ...
Organic Chemistry - Unit 2
... substituted hydrocarbons – these are hydrocarbons where at least 1 hydrogen is replaced with some other atom or group of atoms. (substituents) halocarbon – this is a hydrocarbon containing a halogen substituent. alkyl halide – this is an aliphatic hydrocarbon containing halogen substituents. aryl ha ...
... substituted hydrocarbons – these are hydrocarbons where at least 1 hydrogen is replaced with some other atom or group of atoms. (substituents) halocarbon – this is a hydrocarbon containing a halogen substituent. alkyl halide – this is an aliphatic hydrocarbon containing halogen substituents. aryl ha ...
Get Notes - Mindset Learn
... adds bromine water to a sample of each in two different test tubes. She observes that the one compound decolourises the bromine water immediately, whilst the other one only reacts after placing the test tube in direct sunlight. Write down the: ...
... adds bromine water to a sample of each in two different test tubes. She observes that the one compound decolourises the bromine water immediately, whilst the other one only reacts after placing the test tube in direct sunlight. Write down the: ...
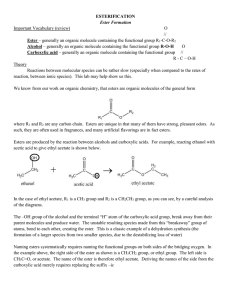
ESTERIFICATION Ester Formation Important Vocabulary (review) O
... In the case of ethyl acetate, R1 is a CH3 group and R2 is a CH2CH3 group, as you can see, by a careful analysis of the diagrams. The –OH group of the alcohol and the terminal “H” atom of the carboxylic acid group, break away from their parent molecules and produce water. The unstable resulting speci ...
... In the case of ethyl acetate, R1 is a CH3 group and R2 is a CH2CH3 group, as you can see, by a careful analysis of the diagrams. The –OH group of the alcohol and the terminal “H” atom of the carboxylic acid group, break away from their parent molecules and produce water. The unstable resulting speci ...
1. All the questions are compulsory. 2. Q. N
... (c) Care for environment, concern for the health of the people or any other two relevant points. ...
... (c) Care for environment, concern for the health of the people or any other two relevant points. ...
Chemistry - CBSE Academic
... The feasibility of thermal reduction can be predicted on the basis of Ellingham diagram. Metals for which the standard free energy of formation ( f Go ) is more negative can reduce those metals for which f Go is less negative. At a given temperature, any metal will reduce the oxide of other meta ...
... The feasibility of thermal reduction can be predicted on the basis of Ellingham diagram. Metals for which the standard free energy of formation ( f Go ) is more negative can reduce those metals for which f Go is less negative. At a given temperature, any metal will reduce the oxide of other meta ...
General Chemistry (II) Chapter 1: Chemical Kinetic 1
... General Chemistry (II) Chapter 1: Chemical Kinetic ...
... General Chemistry (II) Chapter 1: Chemical Kinetic ...
Chapter 13 – Alcohols, Phenols, Ethers, and Thioethers
... bind in only one way before the reaction begins. This ensures only one product comes out and that product may not be the one preferred by standard organic methods. Oxidation In an oxidation one of two things will happen. Either an external reagent will remove two hydrogen atoms (resulting in the con ...
... bind in only one way before the reaction begins. This ensures only one product comes out and that product may not be the one preferred by standard organic methods. Oxidation In an oxidation one of two things will happen. Either an external reagent will remove two hydrogen atoms (resulting in the con ...
Chemical Reactions & Balancing Equations
... number of atoms on both sides. • Use only molecules or atoms already in the formulas • No new compounds used or created • Start with the element you need more of! ...
... number of atoms on both sides. • Use only molecules or atoms already in the formulas • No new compounds used or created • Start with the element you need more of! ...
Name__________________________ Period_______ Word
... (l) – liquid…water is in the liquid state unless otherwise indicated) (g) – gas… all diatomic molecules are gases (H2, N2, F2, Cl2, Br2, Cl2, I2) (aq) – in aqueous solution (dissolved in water)… all acids are in aqueous state ...
... (l) – liquid…water is in the liquid state unless otherwise indicated) (g) – gas… all diatomic molecules are gases (H2, N2, F2, Cl2, Br2, Cl2, I2) (aq) – in aqueous solution (dissolved in water)… all acids are in aqueous state ...
Kinetics of a Reaction
... require students to develop science practice skills involving mathematical reasoning and data analysis. The classic AP chemistry experiment can be transitioned to a guided-inquiry experiment using some or all of the following approaches. These strategies will encourage students to be better prepared ...
... require students to develop science practice skills involving mathematical reasoning and data analysis. The classic AP chemistry experiment can be transitioned to a guided-inquiry experiment using some or all of the following approaches. These strategies will encourage students to be better prepared ...
Chemical reactions cause chemical changes. They involve the
... a change in substances and a change in energy. However, neither matter nor energy is created or destroyed in a chemical reaction. The fact that matter is not created or destroyed in a chemical reaction is called the law of conservation of mass. In order for chemical reaction equations to show that n ...
... a change in substances and a change in energy. However, neither matter nor energy is created or destroyed in a chemical reaction. The fact that matter is not created or destroyed in a chemical reaction is called the law of conservation of mass. In order for chemical reaction equations to show that n ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.