* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemical Reactions & Balancing Equations
Nuclear transmutation wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Double layer forces wikipedia , lookup
Marcus theory wikipedia , lookup
Computational chemistry wikipedia , lookup
Organic chemistry wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
History of chemistry wikipedia , lookup
Biochemistry wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Electrochemistry wikipedia , lookup
Molecular dynamics wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Isotopic labeling wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Click chemistry wikipedia , lookup
George S. Hammond wikipedia , lookup
Chemical bond wikipedia , lookup
Rate equation wikipedia , lookup
Chemical reaction wikipedia , lookup
Transition state theory wikipedia , lookup
Atomic theory wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
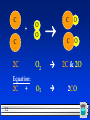
Bell Ringer Active on SOCRATIVE (room:crice) OR Grab a paper copy! Turn it in to the tray and look over the Lab Procedure and today’s notes. 1 Chemical Reactions & Balancing Equations 2 Chemical Reactions 2 Parts: Reactants: substances at the start Products: substances at the end The reactants turn into the products. Reactants Products 3 Equation Format: “+” means “reacts with” or “and” “” means “yields” or “forms” Reactants written on LEFT side Products written on RIGHT side 4 Inquiry Lab Effervescent Tablet –Na(HCO3) & C6H8O7 NaHCO3+ C6H8O7 Na3C6H8O7 + H2O + CO2 sodium + citric sodium + water + carbon bicarbonate acid citrate dioxide 5 In a Chemical Reaction The way atoms are joined is changed Atoms are neither created nor destroyed. –This is the Law of Conservation of Matter 6 Balanced Equation: – Each element has same # of atoms on both sides of the equation. C C + O O + O2 O C O CO2 This equation is already balanced – same number of atoms of each type of element on each side What if it isn’t balanced already? 7 If it is NOT Balanced Already… C + C + O O O2 C O CO • This is called a skeletal reaction • Shows all the reactants and products • Does not show balanced quantities 8 Cooking Analogy for Chemical Reactions: Skeletal reaction: – Ingredients & description of meal Balanced reaction: – Exact recipe 9 Skeletal Reaction Balanced Equation C + O O C O C O • Add molecules until there is an equal number of atoms on both sides. • Use only molecules or atoms already in the formulas • No new compounds used or created • Start with the element you need more of! 10 Skeletal Reaction Balanced Equation C 1C + O O O2 C O C O 2C & 2O • The 2 subscript on Oxygen means there are 2 of them in the molecule. • Still not balanced need 1 more C on the reactant side. 11 C + C 2C O O C O C O O2 2C & 2O O2 2CO Equation: 2C 12 + C + C O O C O C O 2C + O2 2CO Coefficient: •Large Number left of the compound formula. •Represents the number of molecules/atoms • Helps to balance the equations • makes # of atoms on left side = # on right side 13 • Coefficients & Subscripts on the same molecule • The numbers must be multiplied to determine the number of atoms of that element. 2C 2C + 2O2 4O 2CO2 2C & 4O • Notice the subscript only applies to the element it is next to! 14