Chapter 8powerp point for chemical reactions
... Solid calcium metal reacts with water to form aqueous calcium hydroxide and hydrogen gas. ...
... Solid calcium metal reacts with water to form aqueous calcium hydroxide and hydrogen gas. ...
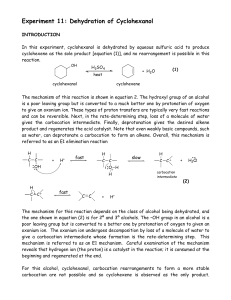
Dehydration of Cyclohexanol
... carbocations derived from certain 2°alcohols may undergo rearrangement to form more stable carbocations. This can result in the formation of rearranged isomeric alkenes. Both 2° and 3° alcohols primarily undergo the E1 reaction under these conditions, whereas for 1° alcohols and methyl alcohol, symm ...
... carbocations derived from certain 2°alcohols may undergo rearrangement to form more stable carbocations. This can result in the formation of rearranged isomeric alkenes. Both 2° and 3° alcohols primarily undergo the E1 reaction under these conditions, whereas for 1° alcohols and methyl alcohol, symm ...
Organic Chemistry I Laboratory
... questioned by two other researchers, John J. Cawley and Patrick E. Lindner. Cawley and Lindner proposed an “E2-like” mechanism involving bridged ions (J. Chem. Educ. 1997, 74, 102.), but it remains to be seen whether their mechanism will gain general acceptance. A mechanism is, after all, a scientif ...
... questioned by two other researchers, John J. Cawley and Patrick E. Lindner. Cawley and Lindner proposed an “E2-like” mechanism involving bridged ions (J. Chem. Educ. 1997, 74, 102.), but it remains to be seen whether their mechanism will gain general acceptance. A mechanism is, after all, a scientif ...
2. ACTIVATION OF CARBOXYL GROUPS IN
... an N-acylated amino acid was protected by conversion to an oxazole derivative, which on photooxygenation regenerated the carboxyl group in the activated triamide form.7 8 ...
... an N-acylated amino acid was protected by conversion to an oxazole derivative, which on photooxygenation regenerated the carboxyl group in the activated triamide form.7 8 ...
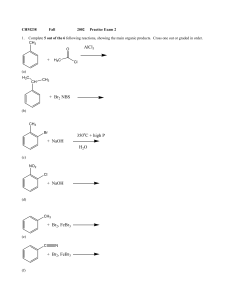
+ NaOH 350 C + high P H2O + H3C AlCl3 + NaOH + Br2, FeBr3
... 10. What would be the product of (a) 1-propanol + PBr3 Æ (b) cyclohexanol + POCl3 Æ (c) (S)-2-butanol + TosCl/pyridine followed by NaBr in DMF? (d) 1-propanol + Jones Reagent (CrO3-H2SO4) Æ (e) 1-propanol + PCC Æ 11. Predict the products of the reaction of Phenyl MgBr with the carbonyl compounds bel ...
... 10. What would be the product of (a) 1-propanol + PBr3 Æ (b) cyclohexanol + POCl3 Æ (c) (S)-2-butanol + TosCl/pyridine followed by NaBr in DMF? (d) 1-propanol + Jones Reagent (CrO3-H2SO4) Æ (e) 1-propanol + PCC Æ 11. Predict the products of the reaction of Phenyl MgBr with the carbonyl compounds bel ...
Carbonyl Condensation Reactions
... If the propanal is reacted with LDA and quickly converted (almost) completely to enolate anion, it will not be able to undergo an aldol reaction. The enolate can then be added to benzyl bromide to form 2-methyl-3-phenylpropanal. On the other hand, if a small amount of KOH is added to a mixture of pr ...
... If the propanal is reacted with LDA and quickly converted (almost) completely to enolate anion, it will not be able to undergo an aldol reaction. The enolate can then be added to benzyl bromide to form 2-methyl-3-phenylpropanal. On the other hand, if a small amount of KOH is added to a mixture of pr ...
( +)-Limonene Oxidation with Selenium Dioxide
... studied by several workers,2a-d and the products identified involved oxidation a t all allylic positions except carbon-3 (menthol series). Most of these oxidation products are constituents of natural products such as citrus essential oils,2e oi which (+)-limonene is the major constituent. As part of ...
... studied by several workers,2a-d and the products identified involved oxidation a t all allylic positions except carbon-3 (menthol series). Most of these oxidation products are constituents of natural products such as citrus essential oils,2e oi which (+)-limonene is the major constituent. As part of ...
Faculteit der Natuurwetenschappen, Wiskunde en Informatica
... A new approach towards the right-hand side of Solanoeclepin A (figure 3) is under investigation. The key step in the synthesis of the tricyclic core of the natural product is a [2+2] photocycloaddition. A challenging part of this approach is to obtain enantiomerically pure β-hydroxy-ketones (17, fig ...
... A new approach towards the right-hand side of Solanoeclepin A (figure 3) is under investigation. The key step in the synthesis of the tricyclic core of the natural product is a [2+2] photocycloaddition. A challenging part of this approach is to obtain enantiomerically pure β-hydroxy-ketones (17, fig ...
Biochemistry I (CHE 418 / 5418)
... each element are on the left as on the right. – Add coefficients in FRONT of chemical formula; NEVER change subscripts on formulas – Start by balancing an element that appears in only one species on each ...
... each element are on the left as on the right. – Add coefficients in FRONT of chemical formula; NEVER change subscripts on formulas – Start by balancing an element that appears in only one species on each ...
Carboxylic acids Acyl chlorides Amides Esters
... Using this way, you will clearly see the effect on the shape of the molecule. ...
... Using this way, you will clearly see the effect on the shape of the molecule. ...
organic synthesis
... Chemical synthesis involves the preparation of new compounds from others. Many industrial processes involve a multi stage process where functional groups are converted into other functional groups. When planning a synthetic route, chemists must consider... • the reagents required to convert one func ...
... Chemical synthesis involves the preparation of new compounds from others. Many industrial processes involve a multi stage process where functional groups are converted into other functional groups. When planning a synthetic route, chemists must consider... • the reagents required to convert one func ...
2009
... 1 Check that the answer sheet provided is for Chemistry Higher (Section A). 2 For this section of the examination you must use an HB pencil and, where necessary, an eraser. 3 Check that the answer sheet you have been given has your name, date of birth, SCN (Scottish Candidate Number) and Centre Name ...
... 1 Check that the answer sheet provided is for Chemistry Higher (Section A). 2 For this section of the examination you must use an HB pencil and, where necessary, an eraser. 3 Check that the answer sheet you have been given has your name, date of birth, SCN (Scottish Candidate Number) and Centre Name ...
Chemistry Final - Practice Test I
... (c) Formation of a new substance (d) Change in density Circle the chemical changes below Burning paper Melting Ice C6H12O6 + H2O C6H12O6 + H2O Ca + H2O CaO + H2 Identify the following as an element, compound, or mixture. ...
... (c) Formation of a new substance (d) Change in density Circle the chemical changes below Burning paper Melting Ice C6H12O6 + H2O C6H12O6 + H2O Ca + H2O CaO + H2 Identify the following as an element, compound, or mixture. ...
3.5 The Alcohols
... 5) An unknown alcohol with molecular formula C3H8O was oxidised using K2Cr2O7 / H2SO The oxidation product collected and tested: The oxidation product was added to AgNO3 dissolved in ammonia and heated. The inside of the tube gave a silver precipitate. a. What functional group must the oxidation pr ...
... 5) An unknown alcohol with molecular formula C3H8O was oxidised using K2Cr2O7 / H2SO The oxidation product collected and tested: The oxidation product was added to AgNO3 dissolved in ammonia and heated. The inside of the tube gave a silver precipitate. a. What functional group must the oxidation pr ...
Answers
... molecular formula. (c) Why do different compounds with the same empirical formula not all have identical chemical properties? (a) Acetylene (ethyne), like benzene, has the empirical formula CH. (b) The molecular formula shows the actual number of atoms of each type in the molecule. The empirical for ...
... molecular formula. (c) Why do different compounds with the same empirical formula not all have identical chemical properties? (a) Acetylene (ethyne), like benzene, has the empirical formula CH. (b) The molecular formula shows the actual number of atoms of each type in the molecule. The empirical for ...
1.4 Alcohols, Ethers and Thiols Answers
... 5. The equation that represents the complete combustion of methanol is: 2 CH3OH(l) + 3 O2(g) → 2 CO2(g) + 4 H2O(g) 6. Answers may vary. Sample answer: The chemist can react ethene with water using an acid as a catalyst to change ethene to ethanol. She can also react pent-1-ene with water using an ac ...
... 5. The equation that represents the complete combustion of methanol is: 2 CH3OH(l) + 3 O2(g) → 2 CO2(g) + 4 H2O(g) 6. Answers may vary. Sample answer: The chemist can react ethene with water using an acid as a catalyst to change ethene to ethanol. She can also react pent-1-ene with water using an ac ...
Synthesis of hetero cyclic compounds pyrazole and pyridiazine from
... stretching band of NH. The 1H NMR (DMSO_d6) of compound [2] : 6.5(d, 4H , CH aromatic ), 6.4(t, 2H , CH aromatic ), 8.5 (s , 1H,NH) , 1.4 , 1.5 (2t, 2(2H), CH 2CH2) ,4.2 (s, 2H, CH2), 2.2 (d, 2H, CH2),5.3 (s ,2H,NH2), 2.0 (3s , 3(3H), CH3). Pyridazine derivatives have been preparation by treatment o ...
... stretching band of NH. The 1H NMR (DMSO_d6) of compound [2] : 6.5(d, 4H , CH aromatic ), 6.4(t, 2H , CH aromatic ), 8.5 (s , 1H,NH) , 1.4 , 1.5 (2t, 2(2H), CH 2CH2) ,4.2 (s, 2H, CH2), 2.2 (d, 2H, CH2),5.3 (s ,2H,NH2), 2.0 (3s , 3(3H), CH3). Pyridazine derivatives have been preparation by treatment o ...
Full Text - Iraqi National Journal of Chemistry
... stretching band of NH. The 1H NMR (DMSO_d6) of compound [2] : 6.5(d, 4H , CH aromatic ), 6.4(t, 2H , CH aromatic ), 8.5 (s , 1H,NH) , 1.4 , 1.5 (2t, 2(2H), CH 2CH2) ,4.2 (s, 2H, CH2), 2.2 (d, 2H, CH2),5.3 (s ,2H,NH2), 2.0 (3s , 3(3H), CH3). Pyridazine derivatives have been preparation by treatment o ...
... stretching band of NH. The 1H NMR (DMSO_d6) of compound [2] : 6.5(d, 4H , CH aromatic ), 6.4(t, 2H , CH aromatic ), 8.5 (s , 1H,NH) , 1.4 , 1.5 (2t, 2(2H), CH 2CH2) ,4.2 (s, 2H, CH2), 2.2 (d, 2H, CH2),5.3 (s ,2H,NH2), 2.0 (3s , 3(3H), CH3). Pyridazine derivatives have been preparation by treatment o ...
4. Carbonyl chemistry
... The reducing agents sodium borohydride (NaBH4) and lithium aluminium hydride (LiAlH4) are used to reduce aldehydes and ketones to their corresponding alcohols. In mechanistic terms they are considered a source of the H ion. 1. Outline a mechanism to show the reduction of butanone using H . (5 marks) ...
... The reducing agents sodium borohydride (NaBH4) and lithium aluminium hydride (LiAlH4) are used to reduce aldehydes and ketones to their corresponding alcohols. In mechanistic terms they are considered a source of the H ion. 1. Outline a mechanism to show the reduction of butanone using H . (5 marks) ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.