thiols and sulfides.
... Tertiary ethers are protecting groups for alcohols. Esters containing tertiary alkyl groups react in dilute acid to give carbocations which are either trapped (SN1) by good nucleophiles or deprotonated in the absence of good nucleophiles. ...
... Tertiary ethers are protecting groups for alcohols. Esters containing tertiary alkyl groups react in dilute acid to give carbocations which are either trapped (SN1) by good nucleophiles or deprotonated in the absence of good nucleophiles. ...
Esters, fats and oils
... The first part of the name comes from the alcohol and the second part from the carboxylic acid. So ethyl ethanoate is made from the alcohol - ethanol and the carboxylic acid - ethanoic acid. Notice that in the name, the alcohol part is named first and the carboxylic acid second. ...
... The first part of the name comes from the alcohol and the second part from the carboxylic acid. So ethyl ethanoate is made from the alcohol - ethanol and the carboxylic acid - ethanoic acid. Notice that in the name, the alcohol part is named first and the carboxylic acid second. ...
Summary of 5.4
... nitration and Friedel-Crafts reactions including the formation of the electrophile 5.4.1 c Heterolytic, electrophilic substitution (benzene) Electrophilic substitution is possible in benzene rings. In this type of substitution two of the delocalised [pi] electrons on the benzene ring are ...
... nitration and Friedel-Crafts reactions including the formation of the electrophile 5.4.1 c Heterolytic, electrophilic substitution (benzene) Electrophilic substitution is possible in benzene rings. In this type of substitution two of the delocalised [pi] electrons on the benzene ring are ...
2.1 Molecules and Metabolism
... bonds an atom will form with other atoms • Remember the OCTET RULE!! All atoms lose/ gain/ share electrons so they can get 8 electrons on their outer shell ...
... bonds an atom will form with other atoms • Remember the OCTET RULE!! All atoms lose/ gain/ share electrons so they can get 8 electrons on their outer shell ...
1 - Intro to Electrochemistry
... Oxidation occurs when a substance _________ electrons during a chemical reaction Its oxidation number _____________________ (more on this later) Example: Cu(s) Cu2+ + 2 eReduction During reduction, a substance ____________ electrons during a chemical reaction The oxidation number of the substance ...
... Oxidation occurs when a substance _________ electrons during a chemical reaction Its oxidation number _____________________ (more on this later) Example: Cu(s) Cu2+ + 2 eReduction During reduction, a substance ____________ electrons during a chemical reaction The oxidation number of the substance ...
The loss of water (dehydration) and the loss of hydrogen are two
... often called dehydrogenation rather than oxidation. Enzymes that catalyze such reactions are called dehydrogenases. Only primary and secondary alcohols can be oxidized by the loss of 2H atoms. Molecules of tertiary alcohols do not have an H atom on the carbon that holds the OH group, so 30 alcohols ...
... often called dehydrogenation rather than oxidation. Enzymes that catalyze such reactions are called dehydrogenases. Only primary and secondary alcohols can be oxidized by the loss of 2H atoms. Molecules of tertiary alcohols do not have an H atom on the carbon that holds the OH group, so 30 alcohols ...
File
... 25 CH3CH2COCH2CH3 reacts with hydrogen cyanide to form an organic product called a cyanohydrin. Which feature applies to the cyanohydrin product? A ...
... 25 CH3CH2COCH2CH3 reacts with hydrogen cyanide to form an organic product called a cyanohydrin. Which feature applies to the cyanohydrin product? A ...
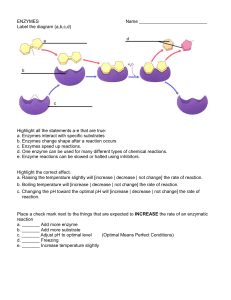
ENZYMES
... Highlight the correct effect. a. Raising the temperature slightly will [increase | decrease | not change] the rate of reaction. b. Boiling temperature will [increase | decrease | not change] the rate of reaction. c. Changing the pH toward the optimal pH will [increase | decrease | not change] the ra ...
... Highlight the correct effect. a. Raising the temperature slightly will [increase | decrease | not change] the rate of reaction. b. Boiling temperature will [increase | decrease | not change] the rate of reaction. c. Changing the pH toward the optimal pH will [increase | decrease | not change] the ra ...
New process of low-temperature methanol synthesis from CO/CO2
... However, similar to the BNL method, in this process CO2 and H2O act as poisons to the catalyst (RONa) and must be completely removed from syngas, making commercialization of low-temperature methanol synthesis impossible. It is well known that for methanol synthesis from CO/CO2/H2 over supported copp ...
... However, similar to the BNL method, in this process CO2 and H2O act as poisons to the catalyst (RONa) and must be completely removed from syngas, making commercialization of low-temperature methanol synthesis impossible. It is well known that for methanol synthesis from CO/CO2/H2 over supported copp ...
Chapter 4-Carbon & Diversity of Life
... Compounds referred to as Thiols Example: the amino acid Cysteine Two of these groups can form a covalent bond and cross link to stabilize protein structure These cross-links also determine the straightness or curliness of hair ...
... Compounds referred to as Thiols Example: the amino acid Cysteine Two of these groups can form a covalent bond and cross link to stabilize protein structure These cross-links also determine the straightness or curliness of hair ...
SCH4C: Chemistry, Grade 12, College Preparation
... c. It has a low electronegativity. d. It has seven valence electrons. 14. Carbon is the backbone of organic molecules because: a. It forms four bonds. b. It has a low electronegativity. c. It is relatively stable. d. It can form chains with itself and still have room for other atoms. e. all of these ...
... c. It has a low electronegativity. d. It has seven valence electrons. 14. Carbon is the backbone of organic molecules because: a. It forms four bonds. b. It has a low electronegativity. c. It is relatively stable. d. It can form chains with itself and still have room for other atoms. e. all of these ...
533548c0-13d7-40fd-8ab5
... 4. Comparatively inert 5. Acidic 6. C2H5OC2H5 7. Diethylether 8. Nucleophilic substitution reaction 9. Peroxide ...
... 4. Comparatively inert 5. Acidic 6. C2H5OC2H5 7. Diethylether 8. Nucleophilic substitution reaction 9. Peroxide ...
Definitions - Loreto Science
... attractions between molecules in which hydrogen atoms are bonded to nitrogen, oxygen or fluorine. • The hydrogen atom carries a partial positive charge and is attracted to the electronegative atom in another molecule. Thus, H acts as a bridge between two electronegative atoms. AG ...
... attractions between molecules in which hydrogen atoms are bonded to nitrogen, oxygen or fluorine. • The hydrogen atom carries a partial positive charge and is attracted to the electronegative atom in another molecule. Thus, H acts as a bridge between two electronegative atoms. AG ...
haloalkanes (halogenoalkanes)
... This form of nucleophilic substitution discussed so far is known as SN2; it is a bimolecular process. An alternative method involves the initial breaking of the C-X bond to form a carbocation, or carbonium ion, (a unimolecular process - SN1 mechanism), which is then attacked by the nucleophile. SN1 ...
... This form of nucleophilic substitution discussed so far is known as SN2; it is a bimolecular process. An alternative method involves the initial breaking of the C-X bond to form a carbocation, or carbonium ion, (a unimolecular process - SN1 mechanism), which is then attacked by the nucleophile. SN1 ...
Lecture 15a - UCLA Chemistry and Biochemistry
... • Acetylene, carbon monoxide and alcohols are reacted in the presence of a catalyst like Ni(CO)4, HCo(CO)4 or Fe(CO)5 to yield acrylic acid esters • If water is used instead of alcohols, the carboxylic acid is obtained (i.e., acrylic acid) • The BHC process to synthesize of ibuprofen uses a palladiu ...
... • Acetylene, carbon monoxide and alcohols are reacted in the presence of a catalyst like Ni(CO)4, HCo(CO)4 or Fe(CO)5 to yield acrylic acid esters • If water is used instead of alcohols, the carboxylic acid is obtained (i.e., acrylic acid) • The BHC process to synthesize of ibuprofen uses a palladiu ...
UNIT 2 - Glow Blogs
... attached, and two have methyl groups attached therefore none of them has four different groups attached. ...
... attached, and two have methyl groups attached therefore none of them has four different groups attached. ...
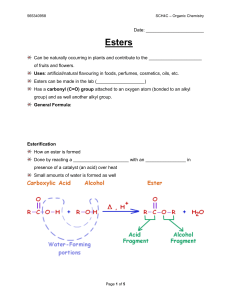
Esters - Mr. Lee`s Science
... Uses: artificial/natural flavouring in foods, perfumes, cosmetics, oils, etc. Esters can be made in the lab (___________________) Has a carbonyl (C=O) group attached to an oxygen atom (bonded to an alkyl group) and as well another alkyl group. General Formula: ...
... Uses: artificial/natural flavouring in foods, perfumes, cosmetics, oils, etc. Esters can be made in the lab (___________________) Has a carbonyl (C=O) group attached to an oxygen atom (bonded to an alkyl group) and as well another alkyl group. General Formula: ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.