* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 533548c0-13d7-40fd-8ab5
Survey
Document related concepts
Transcript
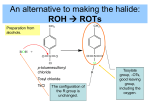
Ethers-book back questions Choose 1. Metamerism 2. C2H5-O-C2H5 3. HI 4. Comparatively inert 5. Acidic 6. C2H5OC2H5 7. Diethylether 8. Nucleophilic substitution reaction 9. Peroxide 10. Williamson’s synthesis Pick out 1.c Halogenated ether on treating with alcohols forms higher ether 2.b the formula of diethyl oxonium chloride is (C2H5)2-O+Cl3. c In Williamson’s synthesis ether is formed using alkoxide and alcohol C. Answer in one or two sentences 1. Write the IUPAc names of a.methoxy ethane b.ethoxy benzene 2.Pg 186 formation of peroxide 3.Pg188 –with Grignard reagent 4.PG 184 wiliamson’s synthesis 5.C2H5 –O- C2H5 + H2O Ethoxy ethane 6.Ethers do not form hydrogen bonding with water Hence they are not soluble in water 7.Pg 189 8.Pg 191 Chemical properties (1) 9. Pg 192 First equation and explanation 10. Pg 190 last equation Answer not exceeding 60 words 1.pg 183 2.184,185 any 3 methods 3.pg 190 4.a.pg 186 b.When diethyl is treated with excess hot conc HI ethyl iodide is formed C2H5 –O C2H5 + 2 HI -------------- 2 C2H5 I + H2O c. Pg 189 d. Pg 188 5.Pg 192 points5 and 6 with equation 6.Pg 191,192 Reaction due to the benzene ring 7.Pg 191 chemical properties (2) 8.In diethyl ether, ether oxygen is capable of forming a co ordinate covalent bond with electron deficient species. Thus diethyl ether forms peroxide by the action of oxygen. C2 H5-O C2H5 + O ------------ C2H5 –O-C2H5 l O Whereas in anisole the oxygen is not able to form co ordinate covalent bond with oxygen because of delocalisation of π electrons in benzene ring. Due to delocalisation the electron present on the oxygen is not readily available for donation. The carbon oxygen bond assumes some double bond character, that is the C-O bond becomes strong. So anisole does not form peroxide easily.