DEHYDRATION OF 2-METHYLCYCLOHEXANOL
... mantle until the product distills out. The boiling point of the distillate should be kept below 96oC by regulating the rate of heating3. The efficiency of fractional distillation cannot be maintained if the reaction flask is heated intermittently or unevenly. Continue distilling until 6-8 mL of liqu ...
... mantle until the product distills out. The boiling point of the distillate should be kept below 96oC by regulating the rate of heating3. The efficiency of fractional distillation cannot be maintained if the reaction flask is heated intermittently or unevenly. Continue distilling until 6-8 mL of liqu ...
cbse class – x science solutions
... Show the formation of the compound by a combination of element C with chlorine with the help of electronic structure. (v) What would be the ratio of number of combining atoms in a compound formed by the combination of element A with carbon? (vi) Which one of the given elements is likely to have the ...
... Show the formation of the compound by a combination of element C with chlorine with the help of electronic structure. (v) What would be the ratio of number of combining atoms in a compound formed by the combination of element A with carbon? (vi) Which one of the given elements is likely to have the ...
Slide 1
... not give a very clear picture of what actually occurs in solution. • The complete ionic equation, better represents the actual forms of the reactants and products in ...
... not give a very clear picture of what actually occurs in solution. • The complete ionic equation, better represents the actual forms of the reactants and products in ...
Experiment 15: Reduction and Oxidation of Organic Compounds
... Review Chapter 21 in LTOC, which discusses Infrared Spectroscopy, pp 311-344. Click on Fig. 2, Expt. 15 to find the infrared spectrum of isoborneol. Place a spatula-tip amount of your product in a small test tube. Add a minimum number of drops of dichloromethane, CH2Cl2, and stir to dissolve the sol ...
... Review Chapter 21 in LTOC, which discusses Infrared Spectroscopy, pp 311-344. Click on Fig. 2, Expt. 15 to find the infrared spectrum of isoborneol. Place a spatula-tip amount of your product in a small test tube. Add a minimum number of drops of dichloromethane, CH2Cl2, and stir to dissolve the sol ...
QuickStudy - Organic Chemistry Fundamentals
... Organic reactions: use Bronsted-Lowry and Lewis models ...
... Organic reactions: use Bronsted-Lowry and Lewis models ...
Welcome to 3FF3! Bio
... • ΔS is unfavorable → complex is organized 3 H-bonds overcome the entropy of complex formation • **Note: In synthetic DNAs other interactions can occur ...
... • ΔS is unfavorable → complex is organized 3 H-bonds overcome the entropy of complex formation • **Note: In synthetic DNAs other interactions can occur ...
Name______________________ Period________
... 49. What is the formula for ammonium carbonate? A) NH4(CO3)2 50. What is the name of NaClO2? A) Sodium perchlorate chloride ...
... 49. What is the formula for ammonium carbonate? A) NH4(CO3)2 50. What is the name of NaClO2? A) Sodium perchlorate chloride ...
+ H 2 O(g)
... Info on Decomp Reactions • Energy is usually need to make these reactions happen • Often hard to predict products unless the substance breaks into its ...
... Info on Decomp Reactions • Energy is usually need to make these reactions happen • Often hard to predict products unless the substance breaks into its ...
Yearly Lesson Plan 2007
... explain with examples oxidation and reduction processes in terms of electron transfer, explain with examples oxidising and reducing agents in redox reactions, write oxidation and reduction halfequations and ionic equations. 3.2 Analysing rusting as a redox reaction state the conditions for ...
... explain with examples oxidation and reduction processes in terms of electron transfer, explain with examples oxidising and reducing agents in redox reactions, write oxidation and reduction halfequations and ionic equations. 3.2 Analysing rusting as a redox reaction state the conditions for ...
organic revision nots
... 30. Out of ethanol and propanol, ethanol gives iodoform test whereas propanol does not do so. 31. The reactivity of all the three classes of alcohols with Lucas reagent is different. 32. In aryl alkyl ethers (i) the alkoxy group activates thebenzene ring towards electrophilic substitution and (ii) i ...
... 30. Out of ethanol and propanol, ethanol gives iodoform test whereas propanol does not do so. 31. The reactivity of all the three classes of alcohols with Lucas reagent is different. 32. In aryl alkyl ethers (i) the alkoxy group activates thebenzene ring towards electrophilic substitution and (ii) i ...
Reactions to functionalize benzene
... that it doesn’t completely deactivate the ring (exception is Friedel-Crafts rxn) Take advantage of methods to convert activating to deactivating groups afterwards, for example CH3 to COOH or NH2 to NO2 Example: a multi-step synthesis of p-nitrobenzoic acid ...
... that it doesn’t completely deactivate the ring (exception is Friedel-Crafts rxn) Take advantage of methods to convert activating to deactivating groups afterwards, for example CH3 to COOH or NH2 to NO2 Example: a multi-step synthesis of p-nitrobenzoic acid ...
Summary of Reactions Which Will Appear on Exams
... X = OH, H2SO4 catalyst required (hydration) Markovnikov addition (you need to know the mechanism) 5. ADDITION OF X2 TO ALKENES H ...
... X = OH, H2SO4 catalyst required (hydration) Markovnikov addition (you need to know the mechanism) 5. ADDITION OF X2 TO ALKENES H ...
Give reasons for the following: (i) Bond enthalpy of F2
... Iodoform Test: Ethanal gives iodoform test. CH3CHO + 4NaOH + 3I2 → CHI3 (Yellow ppt.) + HCOONa + 3NaI + 3H2O Propanal does not give this test. CH3CH2CHO + 4NaOH + 3I2 → No Reaction. Distinguish test between Benzoic acid and Phenol: ...
... Iodoform Test: Ethanal gives iodoform test. CH3CHO + 4NaOH + 3I2 → CHI3 (Yellow ppt.) + HCOONa + 3NaI + 3H2O Propanal does not give this test. CH3CH2CHO + 4NaOH + 3I2 → No Reaction. Distinguish test between Benzoic acid and Phenol: ...
Chapter 18 Reactions of aromatics
... conditions that reduce alkene double bonds • Can selectively reduce an alkene double bond in the presence of an aromatic ring • Reduction of an aromatic ring requires more powerful reducing conditions (high pressure or rhodium catalysts) ...
... conditions that reduce alkene double bonds • Can selectively reduce an alkene double bond in the presence of an aromatic ring • Reduction of an aromatic ring requires more powerful reducing conditions (high pressure or rhodium catalysts) ...
Chapter 4:Chemical Quantities and Aqueous Reactions:
... 16. Crude gunpowders often contain a mixture of solid potassium nitrate and charcoal (solid carbon). When such a mixture is heated until a reaction occurs, a solid residue of potassium carbonate is produced. The explosive force of the gunpowder comes from the fact that two gases are also produced, c ...
... 16. Crude gunpowders often contain a mixture of solid potassium nitrate and charcoal (solid carbon). When such a mixture is heated until a reaction occurs, a solid residue of potassium carbonate is produced. The explosive force of the gunpowder comes from the fact that two gases are also produced, c ...
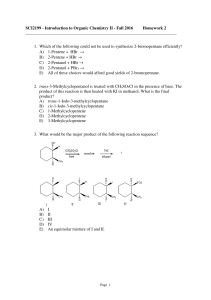
SCI2199 - Introduction to Organic Chemistry II
... 7. Which of the following could be used to synthesize 2-bromobutane? A) CH3CH2CH=CH2 + Br2(aq) → B) CH3CH2CHOHCH3 + HBr → C) CH3CH2C≡CH + HBr → D) CH3CH2C≡CH + Br2 → E) More than one of these choices. 8. Which of the alcohols listed below would you expect to react most rapidly with ...
... 7. Which of the following could be used to synthesize 2-bromobutane? A) CH3CH2CH=CH2 + Br2(aq) → B) CH3CH2CHOHCH3 + HBr → C) CH3CH2C≡CH + HBr → D) CH3CH2C≡CH + Br2 → E) More than one of these choices. 8. Which of the alcohols listed below would you expect to react most rapidly with ...
Name: 1) What is the oxidation number of sulfur in H SO ? A)
... A) Two electrons are gained. B) Two protons are lost. ...
... A) Two electrons are gained. B) Two protons are lost. ...
ORGANIC CONVERSION---(2 to 3 marks)
... (c) Reaction of propanone with methylmagnesium bromide followed by hydrolysis. # Give equations of the following reactions: (i) Oxidation of propan-1-ol with alkaline KMnO4 solution. (ii) Bromine in CS2 with phenol. (iii) Dilute HNO3 with phenol. # Explain the following with an example. Write equati ...
... (c) Reaction of propanone with methylmagnesium bromide followed by hydrolysis. # Give equations of the following reactions: (i) Oxidation of propan-1-ol with alkaline KMnO4 solution. (ii) Bromine in CS2 with phenol. (iii) Dilute HNO3 with phenol. # Explain the following with an example. Write equati ...
Lecture 2 - Chemistry at Winthrop University
... – They are due on the date posted on the class schedule ...
... – They are due on the date posted on the class schedule ...
Your Instructor
... alkenes, alkynes, aromatics); b) name alcohols, phenols, ethers, and amines; c) name acids, aldehydes, ketones, and ethers; d) identify alkanes, alkenes, alkynes and aromatics from structural formulas; e) identify acids, aldehydes, ketones, and esters from structural formulas; f) identify alcohols, ...
... alkenes, alkynes, aromatics); b) name alcohols, phenols, ethers, and amines; c) name acids, aldehydes, ketones, and ethers; d) identify alkanes, alkenes, alkynes and aromatics from structural formulas; e) identify acids, aldehydes, ketones, and esters from structural formulas; f) identify alcohols, ...
aldehyde ketone
... The parent name is the same as that of an alkane, just remove the final “-e” at the end and add “-one”. Unlike the aldehyde, a number MUST be included for the position of the carbonyl carbon in the chain. The number is inserted just before the parent or in front of the suffix ...
... The parent name is the same as that of an alkane, just remove the final “-e” at the end and add “-one”. Unlike the aldehyde, a number MUST be included for the position of the carbonyl carbon in the chain. The number is inserted just before the parent or in front of the suffix ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.