Chapter 7
... Hydrogenation of Alkenes • Reactions where both the catalyst and the substrate are soluble are called Homogeneous catalysis • Rhodium and Ruthenium complexes with a various phosphorus ligands are used in homogeneous catalysis • As we have seen, these types of reactions are called Hydrogenation and ...
... Hydrogenation of Alkenes • Reactions where both the catalyst and the substrate are soluble are called Homogeneous catalysis • Rhodium and Ruthenium complexes with a various phosphorus ligands are used in homogeneous catalysis • As we have seen, these types of reactions are called Hydrogenation and ...
Ethanol as a solvent
... describe the addition of water to ethylene resulting in the production of ethanol and identify the need for a catalyst in this process and the catalyst used process information from secondary sources such as molecular model kits, digital technologies or computer simulations to model ...
... describe the addition of water to ethylene resulting in the production of ethanol and identify the need for a catalyst in this process and the catalyst used process information from secondary sources such as molecular model kits, digital technologies or computer simulations to model ...
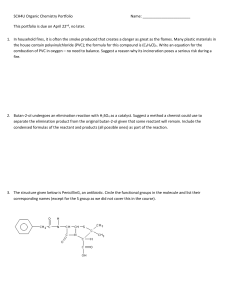
SCH4U Organic Chemistry Portfolio Name: This portfolio is due on
... 5. Nomex is a polymer used to make flame-resistant clothing for firefighters. A portion of its structure is provided below. Write a polymerization reaction showing its production from monomers. What type of reaction is this? ...
... 5. Nomex is a polymer used to make flame-resistant clothing for firefighters. A portion of its structure is provided below. Write a polymerization reaction showing its production from monomers. What type of reaction is this? ...
1 5.03, Inorganic Chemistry Prof. Daniel G. Nocera Lecture 4 Apr 11
... density polyethylene (HDPE, R = H), linear low density polyethylene (LLDPE, R = mostly H with some Et, Bu or Hx), polypropylene (R = Me) and ethylene-propylenediene-modified rubber (EPDM, R = H, Me and alkenyl). ...
... density polyethylene (HDPE, R = H), linear low density polyethylene (LLDPE, R = mostly H with some Et, Bu or Hx), polypropylene (R = Me) and ethylene-propylenediene-modified rubber (EPDM, R = H, Me and alkenyl). ...
Module: 3 Lecture: 15 BUTYL ALCOHOL
... atoms. It belongs to the higher alcohols and branched-chain alcohols. Butanol can be produced by fermentation of biomass by bacteria. When produced biologically called as bio-butanol. It is primarily used as a solvent, as an intermediate in chemical synthesis, and as a fuel. There are four isomeric ...
... atoms. It belongs to the higher alcohols and branched-chain alcohols. Butanol can be produced by fermentation of biomass by bacteria. When produced biologically called as bio-butanol. It is primarily used as a solvent, as an intermediate in chemical synthesis, and as a fuel. There are four isomeric ...
aldehydes powerpoint
... group is always number 1. 3) Identify the branched attachments (alphabetically) and prefix the carbon number it is attached to. If there is more than one of the same type use prefixes. Ex: di for 2, tri for 3 ...
... group is always number 1. 3) Identify the branched attachments (alphabetically) and prefix the carbon number it is attached to. If there is more than one of the same type use prefixes. Ex: di for 2, tri for 3 ...
Document
... surrounded by six electrons (sextet), and is thus electron deficient. The electron-deficient carbene readily adds to an electron rich alkene. ...
... surrounded by six electrons (sextet), and is thus electron deficient. The electron-deficient carbene readily adds to an electron rich alkene. ...
Title - Iowa State University
... 3. Which of the following statements about catalysts is false? a. A catalyst will speed up the rate of a reaction. b. Catalysts are used in very many commercially important chemical reactions. c. Catalytic converters are examples of heterogeneous catalysts. d. A catalyst can cause a nonspontaneous r ...
... 3. Which of the following statements about catalysts is false? a. A catalyst will speed up the rate of a reaction. b. Catalysts are used in very many commercially important chemical reactions. c. Catalytic converters are examples of heterogeneous catalysts. d. A catalyst can cause a nonspontaneous r ...
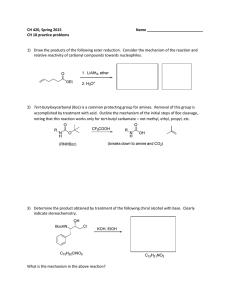
CH 420, Spring 2015 Name ___________________________ CH 18 practice problems
... noting that this reaction works only for tert-butyl carbamate – not methyl, ethyl, propyl, etc. ...
... noting that this reaction works only for tert-butyl carbamate – not methyl, ethyl, propyl, etc. ...
Oxidative Addition
... When the incoming ligand is 13CO, the product contains only one labeled CO, which is cis to the newly formed acetyl group. This shows that the methyl group migrates to a coordinated CO, rather than free CO attacking the Mn−Me bond. We can tell where the labeled CO is located in the product becau ...
... When the incoming ligand is 13CO, the product contains only one labeled CO, which is cis to the newly formed acetyl group. This shows that the methyl group migrates to a coordinated CO, rather than free CO attacking the Mn−Me bond. We can tell where the labeled CO is located in the product becau ...
Name
... Draw the structural formulae for each of the following alcohols and state whether the alcohol is a primary, secondary or tertiary alcohol. (a) Pentan-3-ol. ...
... Draw the structural formulae for each of the following alcohols and state whether the alcohol is a primary, secondary or tertiary alcohol. (a) Pentan-3-ol. ...
HYDROCARBON DERIVATIVES Hydrocarbons are compounds
... What is a 'functional' group? Organic Halides an organic molecule in which one or more of the hydrogens have been replaced with a Group 17 (halogens) atom. Naming Organic halides are named using the same rule as hydrocarbons. The branch is named by shortening the halogen to name to fluoro, chlor ...
... What is a 'functional' group? Organic Halides an organic molecule in which one or more of the hydrogens have been replaced with a Group 17 (halogens) atom. Naming Organic halides are named using the same rule as hydrocarbons. The branch is named by shortening the halogen to name to fluoro, chlor ...
transition metals
... o Desorption of products from the surface of the catalyst Bonding to the surface of the catalyst must be strong enough to weaken bonds and allow reaction to take place but weak enough to allow desorption after the reaction The catalysts with the right amount of adsorption are in the centre of the d- ...
... o Desorption of products from the surface of the catalyst Bonding to the surface of the catalyst must be strong enough to weaken bonds and allow reaction to take place but weak enough to allow desorption after the reaction The catalysts with the right amount of adsorption are in the centre of the d- ...
Alkene Addition Reactions
... The shifting group migrates with its pair of electrons therefore the name hydride (H-‐) or methide (CH3-‐). The order of migrating groups is H > CH3. Alcohols can be produced by addition of ...
... The shifting group migrates with its pair of electrons therefore the name hydride (H-‐) or methide (CH3-‐). The order of migrating groups is H > CH3. Alcohols can be produced by addition of ...
Slides for Chapter 1-4 - Department of Chemistry and Physics
... making the carbon electrophilic Nucleophiles will replace the halide in C-X bonds of many alkyl halides(reaction as Lewis base) Nucleophiles that are Brønsted bases produce ...
... making the carbon electrophilic Nucleophiles will replace the halide in C-X bonds of many alkyl halides(reaction as Lewis base) Nucleophiles that are Brønsted bases produce ...
What is an addition reaction
... In a condensation reaction, two organic molecules react together to produce one larger organic molecule and a molecule of water. For this type of reaction to occur, one of the molecules must have a hydroxyl group, and the other must have an active site with hydrogens, such as another hydroxyl group, ...
... In a condensation reaction, two organic molecules react together to produce one larger organic molecule and a molecule of water. For this type of reaction to occur, one of the molecules must have a hydroxyl group, and the other must have an active site with hydrogens, such as another hydroxyl group, ...
Abstracts - Thieme Verlag
... This chapter provides a concise overview of metal-catalyzed additions to alkenes that involve carbon monoxide and another nucleophilic species, such as water or an alcohol. This is an important area of research in terms of several commodity chemical targets, with many papers devoted to the evolution ...
... This chapter provides a concise overview of metal-catalyzed additions to alkenes that involve carbon monoxide and another nucleophilic species, such as water or an alcohol. This is an important area of research in terms of several commodity chemical targets, with many papers devoted to the evolution ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.