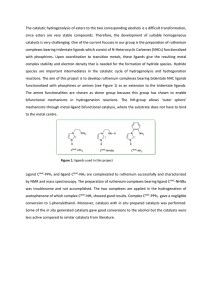
Organometallic Compounds and Catalysis: Synthesis
... Organometallic Compounds and Catalysis: Synthesis and Use of Wilkinson’s Catalyst Organometallic chemistry is the chemistry of compounds which contain a metal carbon bond. Research interest in this area is largely fueled by potential applications of organometallic compounds as catalysts in industria ...
... Organometallic Compounds and Catalysis: Synthesis and Use of Wilkinson’s Catalyst Organometallic chemistry is the chemistry of compounds which contain a metal carbon bond. Research interest in this area is largely fueled by potential applications of organometallic compounds as catalysts in industria ...
Johnson Group Research
... 1) Understanding Current Methodology The most current activation methodology utilizes rhodium complexes to catalyze the cleavage of Csp2-Csp3 single bonds adjacent to ketones, ultimately allowing for the exchange of ketone substituents. This reaction can also be viewed as alkene ‘hydroacylation’, th ...
... 1) Understanding Current Methodology The most current activation methodology utilizes rhodium complexes to catalyze the cleavage of Csp2-Csp3 single bonds adjacent to ketones, ultimately allowing for the exchange of ketone substituents. This reaction can also be viewed as alkene ‘hydroacylation’, th ...
Lecture 13a - University of California, Los Angeles
... • Originally synthesized from IrCl3, triphenylphosphine and various alcohols i.e., 2-methoxyethanol. • Triphenylphosphine as a ligand and reductant in the reaction • A more convenient synthesis uses N,N-dimethylformamide as the CO source (DMF decomposes to CO + HNMe2) • Aniline is frequently used as ...
... • Originally synthesized from IrCl3, triphenylphosphine and various alcohols i.e., 2-methoxyethanol. • Triphenylphosphine as a ligand and reductant in the reaction • A more convenient synthesis uses N,N-dimethylformamide as the CO source (DMF decomposes to CO + HNMe2) • Aniline is frequently used as ...
Lecture 16a
... The reaction was discovered in 1923 The reaction employs hydrogen, carbon monoxide and ...
... The reaction was discovered in 1923 The reaction employs hydrogen, carbon monoxide and ...
Lecture 15a - UCLA Chemistry and Biochemistry
... • The reaction was discovered in 1923 • The reaction employs hydrogen, carbon monoxide and a “metal carbonyl catalyst” to form alkanes, alcohols, etc. • Ruhrchemie A.G. (1936) • Used this process to convert synthesis gas into gasoline using a catalyst Co/ThO2/MgO/Silica gel at 170-200 oC at 1 atm • ...
... • The reaction was discovered in 1923 • The reaction employs hydrogen, carbon monoxide and a “metal carbonyl catalyst” to form alkanes, alcohols, etc. • Ruhrchemie A.G. (1936) • Used this process to convert synthesis gas into gasoline using a catalyst Co/ThO2/MgO/Silica gel at 170-200 oC at 1 atm • ...
$doc.title
... http://www.chem.wisc.edu/areas/clc (Resource page) Reactions of Alcohols #8: Reaction of a 1° Alcohol with Hydrogen Halides ...
... http://www.chem.wisc.edu/areas/clc (Resource page) Reactions of Alcohols #8: Reaction of a 1° Alcohol with Hydrogen Halides ...
The catalytic hydrogenolysis of esters to the two corresponding
... The catalytic hydrogenolysis of esters to the two corresponding alcohols is a difficult transformation, since esters are very stable compounds. Therefore, the development of suitable homogeneous catalysts is very challenging. One of the current focuses in our group is the preparation of ruthenium co ...
... The catalytic hydrogenolysis of esters to the two corresponding alcohols is a difficult transformation, since esters are very stable compounds. Therefore, the development of suitable homogeneous catalysts is very challenging. One of the current focuses in our group is the preparation of ruthenium co ...
Redox Reactions
... an electrophile. They do not react with alkenes or alkynes, so they provide additional selectivity between the functional groups. The reaction mechanism involves the nucleophilic attack of the carbonyl carbon by H-, creating an O-. An acid or aqueous work-up protonates the O- to form the alcohol. So ...
... an electrophile. They do not react with alkenes or alkynes, so they provide additional selectivity between the functional groups. The reaction mechanism involves the nucleophilic attack of the carbonyl carbon by H-, creating an O-. An acid or aqueous work-up protonates the O- to form the alcohol. So ...
C h e m g u i d e ... ALCOHOLS: MANUFACTURE
... 1. One way of manufacturing ethanol is by the direct hydration of ethene with steam. The mixture is passed over a suitable catalyst at a raised temperature and pressure. Only about 5% of the mixture reacts at each pass over the catalyst, and so the ethanol is separated and the unreacted gases passed ...
... 1. One way of manufacturing ethanol is by the direct hydration of ethene with steam. The mixture is passed over a suitable catalyst at a raised temperature and pressure. Only about 5% of the mixture reacts at each pass over the catalyst, and so the ethanol is separated and the unreacted gases passed ...
Chem 30CL-Lecture 15..
... • The reaction was discovered in 1923 • The reaction employs hydrogen, carbon monoxide and a “metal carbonyl catalyst” to form alkanes, alcohols, etc. • Ruhrchemie A.G. (1936) • Used this process to convert synthesis gas into gasoline using a catalyst Co/ThO2/MgO/Silica gel at 170-200 oC at 1 atm • ...
... • The reaction was discovered in 1923 • The reaction employs hydrogen, carbon monoxide and a “metal carbonyl catalyst” to form alkanes, alcohols, etc. • Ruhrchemie A.G. (1936) • Used this process to convert synthesis gas into gasoline using a catalyst Co/ThO2/MgO/Silica gel at 170-200 oC at 1 atm • ...
Synthesis of a Family of Chiral Asymmetric Schiff - Blogs at H-SC
... essential synthetic methods for organic chemists. Condensation reactions of carbonyl compounds are an important class of such reactions. Chiral organometallic compounds have been shown in some cases to act as catalysts to give condensation products in high yields and with high ...
... essential synthetic methods for organic chemists. Condensation reactions of carbonyl compounds are an important class of such reactions. Chiral organometallic compounds have been shown in some cases to act as catalysts to give condensation products in high yields and with high ...
Chem174-Lecture 11a_.. - UCLA Chemistry and Biochemistry
... polynuclear metals carbonyl compounds with an electron deficiency i.e., Rh6(CO)16 (four triply bridged CO groups) Which modes are present in a given compound can often ...
... polynuclear metals carbonyl compounds with an electron deficiency i.e., Rh6(CO)16 (four triply bridged CO groups) Which modes are present in a given compound can often ...
Organometallic Catalysts
... The rate of hydrogenation depends on : (a) presence of a functional group in the vicinity of the C=C bond (b) degree of substitution of the C=C fragment ...
... The rate of hydrogenation depends on : (a) presence of a functional group in the vicinity of the C=C bond (b) degree of substitution of the C=C fragment ...
Alkenes
... The 2s electron and two of the 2p electrons combine to form three sp2 hybrid orbitals, leaving a spare porbital on each of the carbon atoms ...
... The 2s electron and two of the 2p electrons combine to form three sp2 hybrid orbitals, leaving a spare porbital on each of the carbon atoms ...
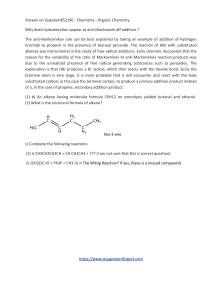
Answer on Question#52196 - Chemistry
... reason for the variability of the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unrealized presence of free radical generating substances such as peroxides. The explanation is that HBr produces a Br radical, which then reacts with the double bond. Since the bromine atom i ...
... reason for the variability of the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unrealized presence of free radical generating substances such as peroxides. The explanation is that HBr produces a Br radical, which then reacts with the double bond. Since the bromine atom i ...
Lecture6-Organometallic Chemistry
... • Homogeneous Catalysis: They are present in the same phase as the reagents • Heterogeneous Catalysts: They are present in a different phase from that of the reactants • Of the two, heterogeneous catalysis has a much ...
... • Homogeneous Catalysis: They are present in the same phase as the reagents • Heterogeneous Catalysts: They are present in a different phase from that of the reactants • Of the two, heterogeneous catalysis has a much ...
The Shell Higher Olefins Process (SHOP)
... 1. Ethene is oligomerized in the presence of the homogeneous nickel catalyst (at 90 – 100°C and 100 – 110 bar) in a polar solvent (1,4-butanediol) to give a mixture of linear, even-numbered αolefins (C4 – C40) with a Flory-Schultz distribution (immiscible with the catalyst solution): ...
... 1. Ethene is oligomerized in the presence of the homogeneous nickel catalyst (at 90 – 100°C and 100 – 110 bar) in a polar solvent (1,4-butanediol) to give a mixture of linear, even-numbered αolefins (C4 – C40) with a Flory-Schultz distribution (immiscible with the catalyst solution): ...
슬라이드 1
... A soluble bis-phosphine complexes, Ni(dppe)2Cl2, is a particularly effective catalyst. The main distinction between this reaction and Pd-catalyzed cross coupling is that the nickel reaction can be more readily applied to saturated alkyl groups because of a reduced tendency for b-elimination. ...
... A soluble bis-phosphine complexes, Ni(dppe)2Cl2, is a particularly effective catalyst. The main distinction between this reaction and Pd-catalyzed cross coupling is that the nickel reaction can be more readily applied to saturated alkyl groups because of a reduced tendency for b-elimination. ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.