Lab 7
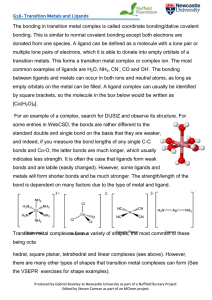
... Figure 6. Oxidation Methodology. We determine that the bond that is most easily oxidized is the C-H bond alpha to the carbonyl group in cyclohexanone. When this bond is cleaved, Step 1, we replace the H atom with an –OH group. The generalized methodology is to place –OH groups on open valences where ...
... Figure 6. Oxidation Methodology. We determine that the bond that is most easily oxidized is the C-H bond alpha to the carbonyl group in cyclohexanone. When this bond is cleaved, Step 1, we replace the H atom with an –OH group. The generalized methodology is to place –OH groups on open valences where ...
study guide
... 39. Hydrogen bonding among individual amino acids in a chain cause what effect on the protein's shape? 40. What is the effect of temperature on protein shape? Give an example of this. 41. Most proteins act as catalysts or __________________ inside of cells. 42. The substance an enzyme is acting upon ...
... 39. Hydrogen bonding among individual amino acids in a chain cause what effect on the protein's shape? 40. What is the effect of temperature on protein shape? Give an example of this. 41. Most proteins act as catalysts or __________________ inside of cells. 42. The substance an enzyme is acting upon ...
Ch 102 – Problem Set 7 Due: Thursday, May 26
... M−L bonds). Label each MO with Mulliken symbols and assign them as σ, σ*, or nb. Populate the MO diagram for a MoV−oxo species. Draw a box around the orbitals of d−parentage. b) For a MoV−oxo species, assign the lowest energy spin−allowed d−d transition. c) Taking into account the π−basicity of oxo ...
... M−L bonds). Label each MO with Mulliken symbols and assign them as σ, σ*, or nb. Populate the MO diagram for a MoV−oxo species. Draw a box around the orbitals of d−parentage. b) For a MoV−oxo species, assign the lowest energy spin−allowed d−d transition. c) Taking into account the π−basicity of oxo ...
Document
... 2HCl(aq) + Cr(s) H2(g)+ CrCl2(aq) A. composition B. single-displacement C. decomposition D. double-displacement ...
... 2HCl(aq) + Cr(s) H2(g)+ CrCl2(aq) A. composition B. single-displacement C. decomposition D. double-displacement ...
REDOX REACTIONS IN ORGANIC CHEMISTRY
... There are a variety of ways for calculating oxidation numbers for organic chemistry. In single C compounds, we use the same (rigorous) procedure used for inorganic chemistry, i.e., assign ox. #’s to all atoms other than C based on their EN values and calculate the ox. # of C from these with the aid ...
... There are a variety of ways for calculating oxidation numbers for organic chemistry. In single C compounds, we use the same (rigorous) procedure used for inorganic chemistry, i.e., assign ox. #’s to all atoms other than C based on their EN values and calculate the ox. # of C from these with the aid ...
No Slide Title - Cobalt
... • The MD simulations show that all the complexes are stable on the free-energy surfaces and do not exhibit ant tendency toward a spontaneous inter-conversion. Further, the complexes are separated by relatively large barriers. In the Ni-case the O p inter-conversion reaction is difficult and leads f ...
... • The MD simulations show that all the complexes are stable on the free-energy surfaces and do not exhibit ant tendency toward a spontaneous inter-conversion. Further, the complexes are separated by relatively large barriers. In the Ni-case the O p inter-conversion reaction is difficult and leads f ...
ORGANIC CHEMISTRY: The chemistry of carbon compounds
... 9. The general formula for an alcohol is: R - OH 10. Benzene is a member of the _____ homologous group. aromatic 11. Alcohols must have this group attached. hydroxyl 12. Longer-chained alcohol like octanol are: not soluble in water and are nonpolar 13 This compound is produced from benzene and is us ...
... 9. The general formula for an alcohol is: R - OH 10. Benzene is a member of the _____ homologous group. aromatic 11. Alcohols must have this group attached. hydroxyl 12. Longer-chained alcohol like octanol are: not soluble in water and are nonpolar 13 This compound is produced from benzene and is us ...
O V O O RO OH t-BuOOH, CH2Cl2, Ti(OPr-i)4(cat), 20 oC (L)
... C bearing the ethyl is the same in each case ((R)-). For the dihydroxylations, however, they function as the complementary systems to get enantiomeric products; the unofficial term pseudoenantiomers is commonly used. It should also be noticed that it is likely the aliphatic nitrogen atom, and not th ...
... C bearing the ethyl is the same in each case ((R)-). For the dihydroxylations, however, they function as the complementary systems to get enantiomeric products; the unofficial term pseudoenantiomers is commonly used. It should also be noticed that it is likely the aliphatic nitrogen atom, and not th ...
Tiebreakers
... Adolph-Strecker amino acid synthesis is one process that creates amino acids naturally from these, and these can be fully reduced to alkanes when they undergo the Wolff-Kishner reduction. Acyloins are a special type of these that react with the Tollen’s reagent, and the Wittig reaction produces alke ...
... Adolph-Strecker amino acid synthesis is one process that creates amino acids naturally from these, and these can be fully reduced to alkanes when they undergo the Wolff-Kishner reduction. Acyloins are a special type of these that react with the Tollen’s reagent, and the Wittig reaction produces alke ...
Page 1 - WordPress.com
... (ii) One isomer of C8H18 is 2,2,3-‐trimethylpentane. Draw the structure of this isomer.(2) (c) State one economic reason for the cracking of petroleum fractions. (1) (d) ( ...
... (ii) One isomer of C8H18 is 2,2,3-‐trimethylpentane. Draw the structure of this isomer.(2) (c) State one economic reason for the cracking of petroleum fractions. (1) (d) ( ...
Steric and Electronic Effects Induced by Ancillary Ligand
... Selective chemical transformation of the hydrocarbon components of natural gas, such as methane, provides solutions to several problems: conversion of natural gas to a liquid would facilitate its transport to remote locations; transformation of methane deposits to benign forms would aid in environme ...
... Selective chemical transformation of the hydrocarbon components of natural gas, such as methane, provides solutions to several problems: conversion of natural gas to a liquid would facilitate its transport to remote locations; transformation of methane deposits to benign forms would aid in environme ...
Ch04 Organic Chem 9e
... 2. What kind of bonds are broken when water vaporizes? 3. If the pH of a lake is 4.0, what is the hydrogen ion [H+] concentration of the lake? What is the hydroxide [OH-] concentration? ...
... 2. What kind of bonds are broken when water vaporizes? 3. If the pH of a lake is 4.0, what is the hydrogen ion [H+] concentration of the lake? What is the hydroxide [OH-] concentration? ...
Summary of Organic chemistry
... Good solvent for other organic cpnds Ethoxyethane (diethyl ether) used as anaesthetic -ethanoic (acetic) acid produced by fermentation of fruit sugar ethanol ethanoic acid (enzyme req'd) -synthesized from ethyne (acetylene) - most esters have pleasant, fruity flavours -natural and synthetic flav ...
... Good solvent for other organic cpnds Ethoxyethane (diethyl ether) used as anaesthetic -ethanoic (acetic) acid produced by fermentation of fruit sugar ethanol ethanoic acid (enzyme req'd) -synthesized from ethyne (acetylene) - most esters have pleasant, fruity flavours -natural and synthetic flav ...
Chapter 4 Warm-Up
... Organic chemistry: branch of chemistry that specializes in study of carbon compounds Organic compounds: contain Carbon (& H) Major elements of life: CHNOPS Carbon can form large, complex, and diverse ...
... Organic chemistry: branch of chemistry that specializes in study of carbon compounds Organic compounds: contain Carbon (& H) Major elements of life: CHNOPS Carbon can form large, complex, and diverse ...
158KB - NZQA
... given time due to having more reactant particles available to collide. This will increase the rate of decomposition of the hydrogen peroxide. ...
... given time due to having more reactant particles available to collide. This will increase the rate of decomposition of the hydrogen peroxide. ...
Chem+174–Lecture4c
... The reference is 2 M Na2MoO4 in water (d=0 ppm) All three compounds exhibit different chemical shifts in the 95Mo-NMR spectrum In all cases, the signals are shifted to more positive values (d= -1100 ppm, -1556 ppm, ?) compared to Mo(CO)6 itself (d=-1857 ppm, CH2Cl2) because the ligands are better s- ...
... The reference is 2 M Na2MoO4 in water (d=0 ppm) All three compounds exhibit different chemical shifts in the 95Mo-NMR spectrum In all cases, the signals are shifted to more positive values (d= -1100 ppm, -1556 ppm, ?) compared to Mo(CO)6 itself (d=-1857 ppm, CH2Cl2) because the ligands are better s- ...
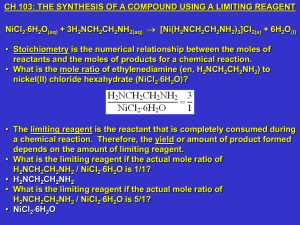
Week #10: The Synthesis of a Compound using a Limiting Reagent
... • Give at least 1 safety concern for the following procedures that will be used in today’s experiment. • Using NiCl26H2O, H2NCH2CH2NH2), and acetone (CH3COCH3). • These are irritants. Wear your goggles at all times. Immediately clean all spills. If you do get either of these in your eye, immediatel ...
... • Give at least 1 safety concern for the following procedures that will be used in today’s experiment. • Using NiCl26H2O, H2NCH2CH2NH2), and acetone (CH3COCH3). • These are irritants. Wear your goggles at all times. Immediately clean all spills. If you do get either of these in your eye, immediatel ...
Document
... • Give at least 1 safety concern for the following procedures that will be used in today’s experiment. • Using NiCl26H2O, H2NCH2CH2NH2), and acetone (CH3COCH3). • These are irritants. Wear your goggles at all times. Immediately clean all spills. If you do get either of these in your eye, immediatel ...
... • Give at least 1 safety concern for the following procedures that will be used in today’s experiment. • Using NiCl26H2O, H2NCH2CH2NH2), and acetone (CH3COCH3). • These are irritants. Wear your goggles at all times. Immediately clean all spills. If you do get either of these in your eye, immediatel ...
File - Mr. J`s Chemistry 4U
... Flourine is the most active halogen. Any metal above magnesium replaces hydrogen from water. Any metal above hydrogen reacts with acids, replacing hydrogen. Elements near the bottom of the activity series are never found free in nature. Elements near the top of the series are often found free in nat ...
... Flourine is the most active halogen. Any metal above magnesium replaces hydrogen from water. Any metal above hydrogen reacts with acids, replacing hydrogen. Elements near the bottom of the activity series are never found free in nature. Elements near the top of the series are often found free in nat ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.