Phenol
... 6) Synthesis of Phenolic acids (Kolbe reaction): Treatment of the salts of a Phenol with carbon dioxide brings about substitution of the carboxyl group, -COOH, for hydrogen of the ring. This reaction is known as the Kolbe reaction; its most important application is in the conversion of Phenol into ...
... 6) Synthesis of Phenolic acids (Kolbe reaction): Treatment of the salts of a Phenol with carbon dioxide brings about substitution of the carboxyl group, -COOH, for hydrogen of the ring. This reaction is known as the Kolbe reaction; its most important application is in the conversion of Phenol into ...
Forward
... Almost half of this methanol is converted to formaldehyde as a starting material for various resins and plastics. Methanol is also used as a solvent, as an antifreeze, and as a convenient clean-burning liquid fuel. This last property makes it a candidate as a fuel for automobiles—methanol is already ...
... Almost half of this methanol is converted to formaldehyde as a starting material for various resins and plastics. Methanol is also used as a solvent, as an antifreeze, and as a convenient clean-burning liquid fuel. This last property makes it a candidate as a fuel for automobiles—methanol is already ...
United States Patent Boyle et aI.
... through Rs have the members necessary to make a porphyrin. chlorin. bacteriochlorin. benzochlorin, hydroxychlorin or hydroxybacteriochlorin nucleus. S1 through S3 are the same or different and can be H. any one of a large number of substituted or unsubstituted alkyl groups, substituted or un substit ...
... through Rs have the members necessary to make a porphyrin. chlorin. bacteriochlorin. benzochlorin, hydroxychlorin or hydroxybacteriochlorin nucleus. S1 through S3 are the same or different and can be H. any one of a large number of substituted or unsubstituted alkyl groups, substituted or un substit ...
Infrared Spectroscopy and Mass Spectroscopy
... ions are bent more than those of heavier atoms. • By varying the magnetic field, the spectrometer plots the abundance of ions of each mass. • The exact radius of curvature of an ion's path depends on its mass-to-charge ratio, symbolized by m/z. In this expression, m is the mass of the ion (in amu) a ...
... ions are bent more than those of heavier atoms. • By varying the magnetic field, the spectrometer plots the abundance of ions of each mass. • The exact radius of curvature of an ion's path depends on its mass-to-charge ratio, symbolized by m/z. In this expression, m is the mass of the ion (in amu) a ...
Disproportionation of Monolithium Acetylide into
... UMR associée au CNRS, campus de Beaulieu, 35042 Rennes Cedex, France Received February 23, 1998 ...
... UMR associée au CNRS, campus de Beaulieu, 35042 Rennes Cedex, France Received February 23, 1998 ...
homogeneous catalysis
... criteria. We have tried to include most of the homogeneous catalytic reactions with proven industrial applications and well-established mechanisms. The basic aim has been to highlight the connections that exist between imaginative academic research and successful technology. In the process, topics a ...
... criteria. We have tried to include most of the homogeneous catalytic reactions with proven industrial applications and well-established mechanisms. The basic aim has been to highlight the connections that exist between imaginative academic research and successful technology. In the process, topics a ...
Solid Manganese Dioxide as an Oxidizing Agent
... acid and concentrated to give a solid which was recrystallized from cyclohexane, 86 mg., m.p. 96-98’ (reportedla for oxyhydrastinine 97-98’). A mixed melting point with an authentic sample, m.p. 97-98’, showed no depression. The acid solution gave 37 mg. of crude unreacted hydrastinine, m . p . 87-9 ...
... acid and concentrated to give a solid which was recrystallized from cyclohexane, 86 mg., m.p. 96-98’ (reportedla for oxyhydrastinine 97-98’). A mixed melting point with an authentic sample, m.p. 97-98’, showed no depression. The acid solution gave 37 mg. of crude unreacted hydrastinine, m . p . 87-9 ...
Some uses of mischmetall in organic synthesis
... The use of rare earth compounds in organic chemistry has grown considerably during the past twenty years. Mainly samarium, cerium, lanthanum, ytterbium, neodymium, dysprosium, lutetium, scandium and yttrium metals and derivatives have been studied. These elements clearly differ in terms of reactivit ...
... The use of rare earth compounds in organic chemistry has grown considerably during the past twenty years. Mainly samarium, cerium, lanthanum, ytterbium, neodymium, dysprosium, lutetium, scandium and yttrium metals and derivatives have been studied. These elements clearly differ in terms of reactivit ...
Improved Synthesis of (3E,6Z,9Z)-1,3,6,9
... of the two species. Thus, a trapping method that is selective for winter moth would be desirable. A geometric isomer of the pheromone, (3E,6Z,9Z)-1,3,6,9-nonadecatetraene (2), can reportedly inhibit attraction of Bruce spanworm to traps without affecting winter moth catch, but use of the pheromone a ...
... of the two species. Thus, a trapping method that is selective for winter moth would be desirable. A geometric isomer of the pheromone, (3E,6Z,9Z)-1,3,6,9-nonadecatetraene (2), can reportedly inhibit attraction of Bruce spanworm to traps without affecting winter moth catch, but use of the pheromone a ...
Document
... Reactions of Alcohols—Dehydration • The E1 dehydration of 20 and 30 alcohols with acid gives clean elimination products without any by-products formed from an SN1 reaction. • Clean elimination takes place because the reaction mixture contains no good nucleophile to react with the intermediate carboc ...
... Reactions of Alcohols—Dehydration • The E1 dehydration of 20 and 30 alcohols with acid gives clean elimination products without any by-products formed from an SN1 reaction. • Clean elimination takes place because the reaction mixture contains no good nucleophile to react with the intermediate carboc ...
Gas-Phase Reactions of Fe (CH2O)+ and Fe (CH2S)+ with Small
... Abstract: The gas-phase reactions of Fe(CH2O)+ and Fe(CH2S)+ with a series of aliphatic alkanes were studied by Fourier transform ion cyclotron resonance (FTICR) mass spectrometry. Like bare Fe+, C-C insertion, particularly terminal C-C insertion, is predominant for the reactions of Fe(CH2O)+, while ...
... Abstract: The gas-phase reactions of Fe(CH2O)+ and Fe(CH2S)+ with a series of aliphatic alkanes were studied by Fourier transform ion cyclotron resonance (FTICR) mass spectrometry. Like bare Fe+, C-C insertion, particularly terminal C-C insertion, is predominant for the reactions of Fe(CH2O)+, while ...
ALCOHOLS AND ETHERS
... (Figure 15-2b). However, there is a relatively broad band around 3350 cm-l, which is characteristic of hydrogen-bonded hydroxyl groups. The shift in frequency of about 300 cm-I arises because hydrogen bonding weakens the 0 - H bond; its absorption frequency then will be lower. The association band i ...
... (Figure 15-2b). However, there is a relatively broad band around 3350 cm-l, which is characteristic of hydrogen-bonded hydroxyl groups. The shift in frequency of about 300 cm-I arises because hydrogen bonding weakens the 0 - H bond; its absorption frequency then will be lower. The association band i ...
Recent developments in the applications of palladium complexes
... salts bearing fluorinated chains can be found in the literature.63-65 In 2000, Xiao and coworkers described the first fluorinated Pd-NHC complex, which was easy to prepare and did not require special handling precautions.65 Aiming to use fluorous biphasic catalysis, the fluorous palladium-NHC comple ...
... salts bearing fluorinated chains can be found in the literature.63-65 In 2000, Xiao and coworkers described the first fluorinated Pd-NHC complex, which was easy to prepare and did not require special handling precautions.65 Aiming to use fluorous biphasic catalysis, the fluorous palladium-NHC comple ...
alcohol - Haverford Alchemy
... • Heating selectively drives off the alkene due to its lower boiling point. • Zaitsev’s Rule states that the more substituted alkene will be favored. This is the result of the equilibrium process that is operating: the less stable form is more likely to revert to the carbocation. © 2013 Pearson Educ ...
... • Heating selectively drives off the alkene due to its lower boiling point. • Zaitsev’s Rule states that the more substituted alkene will be favored. This is the result of the equilibrium process that is operating: the less stable form is more likely to revert to the carbocation. © 2013 Pearson Educ ...
Asymmetric Catalytic Aldol
... • Small amounts of protic additives (alcohols) are critical for catalyst turnover. ...
... • Small amounts of protic additives (alcohols) are critical for catalyst turnover. ...
Catalysts 1
... solvent-free conditions [34]. There have also been reports on the acylation of alcohols using acetic anhydride, catalyzed by silica gel supported Ce(SO4)2, Ti(SO4)2, Fe2(SO4)3, and NaHSO4 [35]. Although the majority of these methods ensure good results, there is still a great need for simple, mild, ...
... solvent-free conditions [34]. There have also been reports on the acylation of alcohols using acetic anhydride, catalyzed by silica gel supported Ce(SO4)2, Ti(SO4)2, Fe2(SO4)3, and NaHSO4 [35]. Although the majority of these methods ensure good results, there is still a great need for simple, mild, ...
Properties of amines
... Amines are compounds based on an ammonia molecule (NH3), where one or more of the hydrogen atoms is replaced by a carbon chain. Thus R—NH2 is a primary amine, while R—NH—R’ is a secondary amine, and R—N(R’)—R’’ is a tertiary amine. You will only be asked to name primary amines. Note that 2-aminopro ...
... Amines are compounds based on an ammonia molecule (NH3), where one or more of the hydrogen atoms is replaced by a carbon chain. Thus R—NH2 is a primary amine, while R—NH—R’ is a secondary amine, and R—N(R’)—R’’ is a tertiary amine. You will only be asked to name primary amines. Note that 2-aminopro ...
OXAZOLINES: THEIR SYNTHESIS AND BIOLOGICAL ACTIVITY
... forms salts with acids and quaternary compounds with alkyl halides. In addition, the 2-oxazoline ring has two sites in the 4 position and two in the 5 position where reactive groups may be located. There are many ways in which oxazoline may be formed. The main interest has been to synthesize 2oxazol ...
... forms salts with acids and quaternary compounds with alkyl halides. In addition, the 2-oxazoline ring has two sites in the 4 position and two in the 5 position where reactive groups may be located. There are many ways in which oxazoline may be formed. The main interest has been to synthesize 2oxazol ...
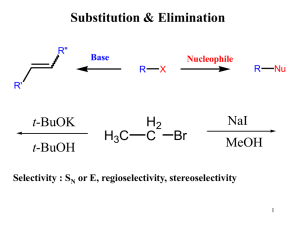
Reactions of Alkyl Halides (SN1, SN2, E1, and E2 reactions)
... elimination. In 3° substrates, only SN1 is possible. In Me° and 1° substrates, SN2 is faster. For 2° substrates, the mechanism of substitution depends upon the solvent. 2. Strong bases, like OH- and OR-, are also good nucleophiles. Substitution and elimination compete. In 3° and 2° alkyl halides, E2 ...
... elimination. In 3° substrates, only SN1 is possible. In Me° and 1° substrates, SN2 is faster. For 2° substrates, the mechanism of substitution depends upon the solvent. 2. Strong bases, like OH- and OR-, are also good nucleophiles. Substitution and elimination compete. In 3° and 2° alkyl halides, E2 ...
Alkene

In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. Alkene, olefin, and olefine are used often interchangeably (see nomenclature section below). Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a homologous series of hydrocarbons with the general formula CnH2n. Alkenes have two hydrogen atoms less than the corresponding alkane (with the same number of carbon atoms). The simplest alkene, ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene is the organic compound produced on the largest scale industrially. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.