Alkyl Halides02
... thionyl chloride, SOCl2, or PBr3) and in this case react via an SN2 mechanism. 3. The Nature of the Nucleophile: The type of Nu:- has great influence on the rate of SN2 reactions but has no influence on SN1 reaction rates because the rate determining step does not involve the Nu: . e.g., t-butanol r ...
... thionyl chloride, SOCl2, or PBr3) and in this case react via an SN2 mechanism. 3. The Nature of the Nucleophile: The type of Nu:- has great influence on the rate of SN2 reactions but has no influence on SN1 reaction rates because the rate determining step does not involve the Nu: . e.g., t-butanol r ...
ETHER
... Has a pair of alkyl or atomic groups attached to a linking oxygen atom. Functional group is ROR Have primary, secondary, and tertiary structures ...
... Has a pair of alkyl or atomic groups attached to a linking oxygen atom. Functional group is ROR Have primary, secondary, and tertiary structures ...
13C -NMR - UCLA Chemistry and Biochemistry
... Protons, neutrons, and electrons all have something called “spin.” This doesn’t mean that they’re actually spinning around in tight circles like Olympian ice skaters, but they’re moving nonetheless and this movement creates a magnetic field around each particle. But for simplicity (and because Chem ...
... Protons, neutrons, and electrons all have something called “spin.” This doesn’t mean that they’re actually spinning around in tight circles like Olympian ice skaters, but they’re moving nonetheless and this movement creates a magnetic field around each particle. But for simplicity (and because Chem ...
CHEM 494 Lecture 8 - UIC Department of Chemistry
... When elimination can occur in more than one direction, the major alkene is the one formed by loss of a H atom from the β carbon having the fewest hydrogens ...
... When elimination can occur in more than one direction, the major alkene is the one formed by loss of a H atom from the β carbon having the fewest hydrogens ...
Topic 5 Energetics File
... Entropy: A measure of the disorder of a system. Things causing entropy to increase: 1) increase of number of moles of gaseous molecules; 2) change of state from solid to liquid or liquid to gas; 3) increase of temperature Exothermic: A reaction in which energy is evolved. ΔH is –. Products more stab ...
... Entropy: A measure of the disorder of a system. Things causing entropy to increase: 1) increase of number of moles of gaseous molecules; 2) change of state from solid to liquid or liquid to gas; 3) increase of temperature Exothermic: A reaction in which energy is evolved. ΔH is –. Products more stab ...
Carbonyl Chemistry (12 Lectures) Aldehydes and Ketones
... – Carbonyl groups in aldehydes and ketones undergo addition reactions. – This is one of the most important reactions of the carbonyl group. ...
... – Carbonyl groups in aldehydes and ketones undergo addition reactions. – This is one of the most important reactions of the carbonyl group. ...
amines
... A nitrogen that bears four substituents is positively charged and is named as an ammonium ion. The anion that is associated with it is also identified in the name. Ammonium salts that have four alkyl groups bonded to nitrogen are called quaternary ammonium salts. ...
... A nitrogen that bears four substituents is positively charged and is named as an ammonium ion. The anion that is associated with it is also identified in the name. Ammonium salts that have four alkyl groups bonded to nitrogen are called quaternary ammonium salts. ...
asymmetric alkyne addition to aldehydes
... Chiral propargylic alcohols are important compounds, as this structural motif is often found in pharmaceutical compounds as well as natural products and can also serve as versatile synthetic intermediates.1 Although there are many methods available for the preparation of these compounds (e.g. asymme ...
... Chiral propargylic alcohols are important compounds, as this structural motif is often found in pharmaceutical compounds as well as natural products and can also serve as versatile synthetic intermediates.1 Although there are many methods available for the preparation of these compounds (e.g. asymme ...
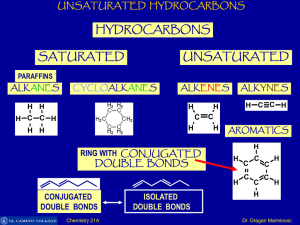
Unsaturated Hydrocarbons
... same plane and their generate a hexagonal ring of C-atoms. Each C-atom in benzene also has an unhybrid 2pz-orbital containing one electron. These 2pz-orbital are perpendicular to the plane of sigma bonds ...
... same plane and their generate a hexagonal ring of C-atoms. Each C-atom in benzene also has an unhybrid 2pz-orbital containing one electron. These 2pz-orbital are perpendicular to the plane of sigma bonds ...
Aldehydes and Ketones
... Naming Aldehydes IUPAC Replace the -e in the alkane name with –al Common Add aldehyde to the prefixes form (1C), acet (2C), propion(3), and butry(4C) O ...
... Naming Aldehydes IUPAC Replace the -e in the alkane name with –al Common Add aldehyde to the prefixes form (1C), acet (2C), propion(3), and butry(4C) O ...
$doc.title
... • Phenol, C6H5OH (“phenyl alcohol”) has diverse uses -‐ it gives its name to the general class of compounds • OH groups bonded to vinylic sp2-‐hybridized carbons are called enols ...
... • Phenol, C6H5OH (“phenyl alcohol”) has diverse uses -‐ it gives its name to the general class of compounds • OH groups bonded to vinylic sp2-‐hybridized carbons are called enols ...
5 Organic Chemistry
... Longer chain hydrocarbons are less useful and therefore less commercially attractive than shorter chain hydrocarbons. Cracking can be used to create shorter hydrocarbons from longer ones. There are two methods of cracking, thermal cracking and catalytic cracking. The following statements could apply ...
... Longer chain hydrocarbons are less useful and therefore less commercially attractive than shorter chain hydrocarbons. Cracking can be used to create shorter hydrocarbons from longer ones. There are two methods of cracking, thermal cracking and catalytic cracking. The following statements could apply ...
review-rough
... 26. The structure given below is a polymer classified as a polyamide. This polymer is stable in temperatures above 350°C, and can be used as a protective coating on hot surfaces. What monomers were used to make this polyamide? ...
... 26. The structure given below is a polymer classified as a polyamide. This polymer is stable in temperatures above 350°C, and can be used as a protective coating on hot surfaces. What monomers were used to make this polyamide? ...
Alcohols and Carbonyls
... 1. Fehlings solution contains Cu2+ ions (blue) which form Cu+ ion (orange-red) in the presence of aldehydes. 2. Tollen’s reagent contains Ag+ ions, which form Ag in the presence of aldehydes (silver mirror test) 3. Acidified Potassium Dichromate orange Cr2O72-(aq) to green Cr3(aq) ...
... 1. Fehlings solution contains Cu2+ ions (blue) which form Cu+ ion (orange-red) in the presence of aldehydes. 2. Tollen’s reagent contains Ag+ ions, which form Ag in the presence of aldehydes (silver mirror test) 3. Acidified Potassium Dichromate orange Cr2O72-(aq) to green Cr3(aq) ...
Part 2 - Class Index
... Below is a list of all substituents that you are responsible for in Chem 2500. Some substituents listed are functional groups that were lower in priority than the principal functional group. ...
... Below is a list of all substituents that you are responsible for in Chem 2500. Some substituents listed are functional groups that were lower in priority than the principal functional group. ...
Aldehydes can react with alcohols to form hemiacetals
... Like their hydrates, the hemiacetals of most ketones (sometimes called hemiketals) are even less stable than those of aldehydes. On the other hand, some hemiacetals of aldehydes bearing electronwithdrawing groups, and those of cyclopropanones, are stable, just like the hydrates of the same molecules ...
... Like their hydrates, the hemiacetals of most ketones (sometimes called hemiketals) are even less stable than those of aldehydes. On the other hand, some hemiacetals of aldehydes bearing electronwithdrawing groups, and those of cyclopropanones, are stable, just like the hydrates of the same molecules ...
High-Oxidation-State Palladium Catalysis: New Reactivity for
... differs significantly, the process itself may be comparable to related reductive elim- Figure 1. Three-center, four-elecination from PdII com- tron transition state for reductive plexes.[2] In the latter case, elimination from s-aryl palladiuthe precise mechanistic pic- m(IV) intermediates in aryl–a ...
... differs significantly, the process itself may be comparable to related reductive elim- Figure 1. Three-center, four-elecination from PdII com- tron transition state for reductive plexes.[2] In the latter case, elimination from s-aryl palladiuthe precise mechanistic pic- m(IV) intermediates in aryl–a ...
Alkene

In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. Alkene, olefin, and olefine are used often interchangeably (see nomenclature section below). Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a homologous series of hydrocarbons with the general formula CnH2n. Alkenes have two hydrogen atoms less than the corresponding alkane (with the same number of carbon atoms). The simplest alkene, ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene is the organic compound produced on the largest scale industrially. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.