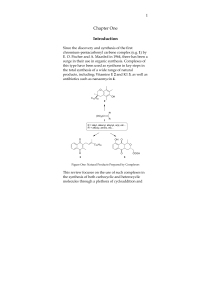
Oxidation
... same). Such as: HBr, HOH, HNO2, HCl, etc 10. Addition of a species Y-Y’ will definitely change the oxidation state of the reaction. Therefore, addition of Y-Y’ (eg. Br-Br) to a double bond is an Oxidation, however, elimination of Y-Y’ from a single bond is reduction. ...
... same). Such as: HBr, HOH, HNO2, HCl, etc 10. Addition of a species Y-Y’ will definitely change the oxidation state of the reaction. Therefore, addition of Y-Y’ (eg. Br-Br) to a double bond is an Oxidation, however, elimination of Y-Y’ from a single bond is reduction. ...
10_OrganicChemistryRC
... Organic Chemistry • 11 2. Add 5 drops of each compound into their labeled test tubes. 3. Add 2 drops of iron (III) chloride solution to each test tube. 4. Note any color changes in each solution and record your observations in the FeCl3 column of your Data Sheet. Based on the data you collected, id ...
... Organic Chemistry • 11 2. Add 5 drops of each compound into their labeled test tubes. 3. Add 2 drops of iron (III) chloride solution to each test tube. 4. Note any color changes in each solution and record your observations in the FeCl3 column of your Data Sheet. Based on the data you collected, id ...
Liquid-gas phase-boundary catalytic system
... heterogeneous catalysts in the liquid-liquid phase boundary [7-18]. In this catalytic reaction system, the catalyst was placed at the liquid-liquid phase boundary between aqueous hydrogen peroxide and water-immiscible organic phases and act as an efficient catalyst for epoxidation reaction. In this ...
... heterogeneous catalysts in the liquid-liquid phase boundary [7-18]. In this catalytic reaction system, the catalyst was placed at the liquid-liquid phase boundary between aqueous hydrogen peroxide and water-immiscible organic phases and act as an efficient catalyst for epoxidation reaction. In this ...
FULL PAPER Observations on the Influence of Precursor
... aldehyde 15 by a one-pot reaction via the diol by treatment first with OsO4 / NMO,26 followed by cleavage of the diol with NaIO4.27 A Grignard reaction using vinylmagnesium bromide in THF at –78 °C afforded the racemic mixture of alcohol 16.20 After protection of the hydroxyl group with TIPS to affo ...
... aldehyde 15 by a one-pot reaction via the diol by treatment first with OsO4 / NMO,26 followed by cleavage of the diol with NaIO4.27 A Grignard reaction using vinylmagnesium bromide in THF at –78 °C afforded the racemic mixture of alcohol 16.20 After protection of the hydroxyl group with TIPS to affo ...
Organic Chemistry II Introduction
... KMnO4 or Na2Cr2O7 gives a substituted benzoic acid 1° and 2° alkyl groups can be oxidized, but tertiary groups do not react ...
... KMnO4 or Na2Cr2O7 gives a substituted benzoic acid 1° and 2° alkyl groups can be oxidized, but tertiary groups do not react ...
19.2 preparation of acyl chlorides
... CH2±O±C±(CH2)7CHœCHCH2CHœCH(CH2)4CH3 H2O NaOH O Conjugate base of X stearic acid ...
... CH2±O±C±(CH2)7CHœCHCH2CHœCH(CH2)4CH3 H2O NaOH O Conjugate base of X stearic acid ...
Latest Publication (still not complete)
... In particular, an analysis of information gained through computational analysis is provided. Such information has been used to rationalize most of the observed reaction pathways and hence is crucial to the total understanding of the chemistry of chromium-pentacarbonyl carbene complexes. Fischer carb ...
... In particular, an analysis of information gained through computational analysis is provided. Such information has been used to rationalize most of the observed reaction pathways and hence is crucial to the total understanding of the chemistry of chromium-pentacarbonyl carbene complexes. Fischer carb ...
CHAPTER 15
... (1) Aldehydes and ketones readily undergo oxidation to carboxylic acids. (2) Propanone and dimethyl ketone are two names for the same compound. (3) The “silver mirror test” distinguishes between aldehydes and ketones. a) All three statements are true. b) Two of the three statements are true. c) Only ...
... (1) Aldehydes and ketones readily undergo oxidation to carboxylic acids. (2) Propanone and dimethyl ketone are two names for the same compound. (3) The “silver mirror test” distinguishes between aldehydes and ketones. a) All three statements are true. b) Two of the three statements are true. c) Only ...
Document
... two less hydrogen atoms. ● Removal of these two hydrogens from a primary alcohol as a result of oxidation yields an aldehyde; where as their removal from a secondary alcohol gives a ketone. ...
... two less hydrogen atoms. ● Removal of these two hydrogens from a primary alcohol as a result of oxidation yields an aldehyde; where as their removal from a secondary alcohol gives a ketone. ...
12602989_294 - University of Canterbury
... perchlorate in toluene solution furnished a completely different product, (6), that was found to be polymeric. This compound9 consists of 1D chains of silver atoms bridged by both η2coordinated meta-divinylbenzene ligands and bidentate perchlorate anions (Figure 3, top). A related assembly (7) was a ...
... perchlorate in toluene solution furnished a completely different product, (6), that was found to be polymeric. This compound9 consists of 1D chains of silver atoms bridged by both η2coordinated meta-divinylbenzene ligands and bidentate perchlorate anions (Figure 3, top). A related assembly (7) was a ...
Page 1 - WordPress.com
... All three are branched-chain molecules and none is cyclic. P can represent a pair of optical isomers. Q can represent a pair of geometrical isomers. R can represent another pair of geometrical isomers different from Q. Draw one possible structure for one of the isomers of each of P, Q and R. (3) But ...
... All three are branched-chain molecules and none is cyclic. P can represent a pair of optical isomers. Q can represent a pair of geometrical isomers. R can represent another pair of geometrical isomers different from Q. Draw one possible structure for one of the isomers of each of P, Q and R. (3) But ...
pdf
... Asymmetric Synthesis • Creates one or more desired chiral centers • Enan)omerically pure chiral catalysts lead to the produc)on of enan)omerically enriched products • 2 func)ons: • Ac)va)ng func)on • Controlling ...
... Asymmetric Synthesis • Creates one or more desired chiral centers • Enan)omerically pure chiral catalysts lead to the produc)on of enan)omerically enriched products • 2 func)ons: • Ac)va)ng func)on • Controlling ...
Organic Chemistry II Introduction
... Thiols do not form H-bonds (EN of S is low) Alcohols and phenols are weakly basic Alcohols and phenols are weakly acidic Phenols and Thiols are more acidic than water ...
... Thiols do not form H-bonds (EN of S is low) Alcohols and phenols are weakly basic Alcohols and phenols are weakly acidic Phenols and Thiols are more acidic than water ...
Aldehydes and Ketones
... • Carbonyl compound: Any compound that contains a carbonyl group, C=O. • Carbonyl group: A functional group that has a C atom joined to an O atom by a double bond. • The bond angles between the three substituents on the carbonyl carbon atom are 120°, or close to it. ...
... • Carbonyl compound: Any compound that contains a carbonyl group, C=O. • Carbonyl group: A functional group that has a C atom joined to an O atom by a double bond. • The bond angles between the three substituents on the carbonyl carbon atom are 120°, or close to it. ...
Chapter Sixteen Aldehydes and Ketones
... C atom form a planar triangle. ► The simplest aldehydes and ketones are known by common names. Aldehydes are named systematically by replacing the final -e in an alkane name with -al. ► Ketones are named systematically by replacing the final -e in an alkane name with -one and numbering starting with ...
... C atom form a planar triangle. ► The simplest aldehydes and ketones are known by common names. Aldehydes are named systematically by replacing the final -e in an alkane name with -al. ► Ketones are named systematically by replacing the final -e in an alkane name with -one and numbering starting with ...
Asymmetric catalytic routes to chiral building blocks of
... Our initial studies in this area were aimed at identifying an efficacious catalyst for enantioselective hydrogenation of substrates 14.l6 Thus, we performed hydrogenations using a series of DuPHOS-Rh and BPE-Rh catalysts under a standard set of reaction conditions (MeOH, 25 O C , 60 psi H2, SIC = 50 ...
... Our initial studies in this area were aimed at identifying an efficacious catalyst for enantioselective hydrogenation of substrates 14.l6 Thus, we performed hydrogenations using a series of DuPHOS-Rh and BPE-Rh catalysts under a standard set of reaction conditions (MeOH, 25 O C , 60 psi H2, SIC = 50 ...
ch221 class 5
... Naming Alkanes Trivial and Systematic Nomenclature The original names of organic compounds are now known as trivial names. These names describe some aspect of the compound, often its origin, and are non-systematic (i.e. they cannot be logically derived from the molecular structure). ...
... Naming Alkanes Trivial and Systematic Nomenclature The original names of organic compounds are now known as trivial names. These names describe some aspect of the compound, often its origin, and are non-systematic (i.e. they cannot be logically derived from the molecular structure). ...
Part A Completion
... © Pearson Education, Inc., publishing as Pearson Prentice Hall. All rights reserved. ...
... © Pearson Education, Inc., publishing as Pearson Prentice Hall. All rights reserved. ...
Synthesis of p-hydroxy alkyl benzoates
... major goal of present research in chemistry. Synthetic esters are generally prepared by reaction of alcohol with organic carboxylic acid in presence of catalyst such as sulphuric acid, the reaction is known as Fischer esterification. The Fischer esterification is a reversible reaction. The equilibri ...
... major goal of present research in chemistry. Synthetic esters are generally prepared by reaction of alcohol with organic carboxylic acid in presence of catalyst such as sulphuric acid, the reaction is known as Fischer esterification. The Fischer esterification is a reversible reaction. The equilibri ...
Pincer Complexes. Applications in Catalysis
... of these complexes have shown activity in the dehydrogenation of alkanes to alkenes. However, the extremely low reaction rates and the low turnover numbers or the instability of the employed catalysts under the reaction conditions16 has limited the use of these species. In 1976 Moulton and Shaw repo ...
... of these complexes have shown activity in the dehydrogenation of alkanes to alkenes. However, the extremely low reaction rates and the low turnover numbers or the instability of the employed catalysts under the reaction conditions16 has limited the use of these species. In 1976 Moulton and Shaw repo ...
Document
... The 3 equivalent hydrogens likely to be methyl group. The two hydrogens a CH2. 5. Have accounted for all atoms but one C and one O. Conclude: Carbonyl group! 6. Absence of splitting between CH2 and CH3. Conclude: they are not adjacent. ...
... The 3 equivalent hydrogens likely to be methyl group. The two hydrogens a CH2. 5. Have accounted for all atoms but one C and one O. Conclude: Carbonyl group! 6. Absence of splitting between CH2 and CH3. Conclude: they are not adjacent. ...
How well do you know your functional groups?
... molecule; particularly the functional groups, but also some aspects of C skeleton Absorption bands can be assigned to certain bonds or functional groups based on three features: frequency, intensity, shape Frequencies: (To aid in assigning IR bands to structural features of a molecule, consult hando ...
... molecule; particularly the functional groups, but also some aspects of C skeleton Absorption bands can be assigned to certain bonds or functional groups based on three features: frequency, intensity, shape Frequencies: (To aid in assigning IR bands to structural features of a molecule, consult hando ...
Alkene

In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. Alkene, olefin, and olefine are used often interchangeably (see nomenclature section below). Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a homologous series of hydrocarbons with the general formula CnH2n. Alkenes have two hydrogen atoms less than the corresponding alkane (with the same number of carbon atoms). The simplest alkene, ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene is the organic compound produced on the largest scale industrially. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.