Oxidation of Diols and Ethers by NaBr03
... Hence, 33 was reacted in water to give 34 in 82% yield (Run 2). It is important that the oxidation proceeds satisfactorily in water, although the conventional oxidation by NaBr03 in the presence of HBr is carried out in CH2C12. The equimolar oxidation of 33 with NaBr03/NaHS03 gave 34 in moderate yie ...
... Hence, 33 was reacted in water to give 34 in 82% yield (Run 2). It is important that the oxidation proceeds satisfactorily in water, although the conventional oxidation by NaBr03 in the presence of HBr is carried out in CH2C12. The equimolar oxidation of 33 with NaBr03/NaHS03 gave 34 in moderate yie ...
Mechanism of the oxymercuration of substituted cyclohexenes
... Stereochemical evidence concerning the possible intermediacy of mercurinium ions has been derived with two distinctly different types of unsaturated systems. Waters and coworkers26have studied the stereochemistry of the oxymercuration of optically active 1,3-dimethylallene, and have compared the ste ...
... Stereochemical evidence concerning the possible intermediacy of mercurinium ions has been derived with two distinctly different types of unsaturated systems. Waters and coworkers26have studied the stereochemistry of the oxymercuration of optically active 1,3-dimethylallene, and have compared the ste ...
catalytic activity of modified silicates: i. dehydration of ethanol
... reported by Brauner& Preisinger (1956). This mineral is constructed of talc-like ribbons arranged in such a way that the tetrahedral sheet is continuous but inverts apical directions in adjacent ribbons, generating channels along the c axis of the fibre. The dimensions of the channels are approximat ...
... reported by Brauner& Preisinger (1956). This mineral is constructed of talc-like ribbons arranged in such a way that the tetrahedral sheet is continuous but inverts apical directions in adjacent ribbons, generating channels along the c axis of the fibre. The dimensions of the channels are approximat ...
topic 6 – hydrocarbons (general level)
... (d) Draw a full structural formula for, an isomer of buta-l,3-diene which contains only one double bond per molecule. 1 mark (PS) ...
... (d) Draw a full structural formula for, an isomer of buta-l,3-diene which contains only one double bond per molecule. 1 mark (PS) ...
The Fischer Indole Synthesis
... Substituents may be added anywhere on the above molecule to create an indole derivative. Several thousand indole derivatives appear annually in chemical literature.3 The Fischer indole synthesis is the most widely used and versatile method for indole synthesis. Reaction Mechanism: The Fischer indole ...
... Substituents may be added anywhere on the above molecule to create an indole derivative. Several thousand indole derivatives appear annually in chemical literature.3 The Fischer indole synthesis is the most widely used and versatile method for indole synthesis. Reaction Mechanism: The Fischer indole ...
Organometallics - X-Ray - University of Kentucky
... and DFT computational studies. It does not appear that 4 possesses agostic interactions in solution. High oxidation state transition metal complexes containing double metal-carbon bonds (usually termed Schrock1 carbenes or alkylidenes) have attracted significant attention,2 in part because of their ...
... and DFT computational studies. It does not appear that 4 possesses agostic interactions in solution. High oxidation state transition metal complexes containing double metal-carbon bonds (usually termed Schrock1 carbenes or alkylidenes) have attracted significant attention,2 in part because of their ...
10 Haloalkanes and Haloarenes
... the bond formed is weaker. Hence, C X bond strength in CH3 X decreases in the order: CH3F > CH3Cl > CH3Br > CH3I as the 2sp3 orbital of carbon cannot penetrate into the larger p-orbitals sufficiently to form strong bonds. ...
... the bond formed is weaker. Hence, C X bond strength in CH3 X decreases in the order: CH3F > CH3Cl > CH3Br > CH3I as the 2sp3 orbital of carbon cannot penetrate into the larger p-orbitals sufficiently to form strong bonds. ...
Compounds Containing a C=O (Carbonyl) Group
... Aldehydes and Ketones Naming Aldehydes, Naming Ketones, Physical Properties, Interesting Aldehydes and Ketones, Preparation of Aldehydes and Ketones, Reactions of Aldehydes and Ketones (Addition of 1° Amines, Addition of 2° Amines, Addition of H2O—Hydration, Addition of Alcohols—Acetal Formation, A ...
... Aldehydes and Ketones Naming Aldehydes, Naming Ketones, Physical Properties, Interesting Aldehydes and Ketones, Preparation of Aldehydes and Ketones, Reactions of Aldehydes and Ketones (Addition of 1° Amines, Addition of 2° Amines, Addition of H2O—Hydration, Addition of Alcohols—Acetal Formation, A ...
CFCs and Alcohols
... aerobically, creating only water and carbon dioxide. The presence of oxygen will also oxidize any ethanol that is made, to form ethanoic acid. Under some circumstances this reaction can be useful, for example, in turning wine into vinegar. ...
... aerobically, creating only water and carbon dioxide. The presence of oxygen will also oxidize any ethanol that is made, to form ethanoic acid. Under some circumstances this reaction can be useful, for example, in turning wine into vinegar. ...
Alcohols, Aldehydes, and Ketones
... of a primary alcohol at the aldehyde stage in order to prevent the aldehyde from being oxidized further to the carboxylic acid. One way to do this is to remove the aldehyde as soon as it is formed by distilling it from the reaction mixture. Reactions such as these can be used to measure alcohol cont ...
... of a primary alcohol at the aldehyde stage in order to prevent the aldehyde from being oxidized further to the carboxylic acid. One way to do this is to remove the aldehyde as soon as it is formed by distilling it from the reaction mixture. Reactions such as these can be used to measure alcohol cont ...
Hein and Arena - faculty at Chemeketa
... In the presence of excess alcohol and a strong acid such as dry HCl, aldehydes or hemiacetals react with a second molecule of the alcohol to yield an acetal. ...
... In the presence of excess alcohol and a strong acid such as dry HCl, aldehydes or hemiacetals react with a second molecule of the alcohol to yield an acetal. ...
Organic Chemistry I PHS 2025 Fall 2013 Section 1 Lecture Topics
... Describing Chemical Bonds: Molecular Orbital Theory Define: Molecular Orbital Theory ...
... Describing Chemical Bonds: Molecular Orbital Theory Define: Molecular Orbital Theory ...
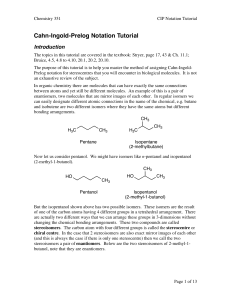
Cahn-Ingold-Prelog Notation Tutorial
... The purpose of this tutorial is to help you master the method of assigning Cahn-IngoldPrelog notation for stereocentres that you will encounter in biological molecules. It is not an exhaustive review of the subject. In organic chemistry there are molecules that can have exactly the same connections ...
... The purpose of this tutorial is to help you master the method of assigning Cahn-IngoldPrelog notation for stereocentres that you will encounter in biological molecules. It is not an exhaustive review of the subject. In organic chemistry there are molecules that can have exactly the same connections ...
C 1 hapter
... adduct C*-B. In addition, there are a wide range of unsaturated substrates, which can react with a borane reagent through transition metal complexes. There are several challenges involved in applying chiral catalysts in the hydroboration reaction: the catalytic performance must be excellent, the dev ...
... adduct C*-B. In addition, there are a wide range of unsaturated substrates, which can react with a borane reagent through transition metal complexes. There are several challenges involved in applying chiral catalysts in the hydroboration reaction: the catalytic performance must be excellent, the dev ...
Handout 3
... • Radical Substitution Reactions, course, stability, and reactivity of radicals • Some free radical reaction mechanisms, Halogenation of alkanes, NBS alllylic substitution, • Free radical addition to alkenes (HBr / Peroxide addition), Radical polymerization of alkenes • Addition Reactions of Alkenes ...
... • Radical Substitution Reactions, course, stability, and reactivity of radicals • Some free radical reaction mechanisms, Halogenation of alkanes, NBS alllylic substitution, • Free radical addition to alkenes (HBr / Peroxide addition), Radical polymerization of alkenes • Addition Reactions of Alkenes ...
Global Challenges - Part 2
... *C6.2a recognise functional groups and identify members of the same homologous series to include homologous series, of alkanes, alkenes, alcohols and carboxylic acids *C6.2b name and draw the structural formulae, using fully displayed formulae, of the first four members of the straight chain alkanes ...
... *C6.2a recognise functional groups and identify members of the same homologous series to include homologous series, of alkanes, alkenes, alcohols and carboxylic acids *C6.2b name and draw the structural formulae, using fully displayed formulae, of the first four members of the straight chain alkanes ...
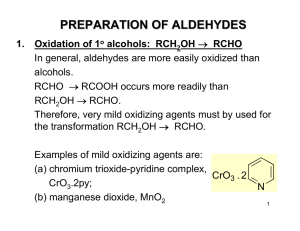
PREPARATION OF ALDEHYDES
... EXAMPLES OF NUCLEOPHILIC ADDITION TO ALDEHYDES & KETONES Addition of HCN (neutral-basic conditions). CN Ө is a very good nucleophile (ionic nucleophile). The use of the actual compound HCN is not experimentally feasible, as it is a lethal gas, bp 26 oC. Addition of the elements of HCN to a C=O grou ...
... EXAMPLES OF NUCLEOPHILIC ADDITION TO ALDEHYDES & KETONES Addition of HCN (neutral-basic conditions). CN Ө is a very good nucleophile (ionic nucleophile). The use of the actual compound HCN is not experimentally feasible, as it is a lethal gas, bp 26 oC. Addition of the elements of HCN to a C=O grou ...
Carbonyl Compounds - Thomas Tallis Science
... Carboxylic acids are named using the suffix –oic acid. Methanoic acid is the simplest carboxylic acid and is found in bee and ant stings. Ethanoic acid is the acid that gives vinegar its sharp taste and smell. It is also important in the chemical industry and about 6.5 million tonnes are used worldw ...
... Carboxylic acids are named using the suffix –oic acid. Methanoic acid is the simplest carboxylic acid and is found in bee and ant stings. Ethanoic acid is the acid that gives vinegar its sharp taste and smell. It is also important in the chemical industry and about 6.5 million tonnes are used worldw ...
Lecture 3 Notes CH.4
... • This allows for the formation of diverse branching (non-linear) structures • Varying the number of bonds also varies the angles of bonds, and therefore, the shapes of molecules – this includes various types of isomers • This allows the incorporation of other atoms (HNOPS) into biological molecules ...
... • This allows for the formation of diverse branching (non-linear) structures • Varying the number of bonds also varies the angles of bonds, and therefore, the shapes of molecules – this includes various types of isomers • This allows the incorporation of other atoms (HNOPS) into biological molecules ...
Worksheets for this unit
... break down these large molecules to useable size is called ___________________. There are two types called ______________ and _________________________. 7. The opposite to cracking is called ____________________________ ...
... break down these large molecules to useable size is called ___________________. There are two types called ______________ and _________________________. 7. The opposite to cracking is called ____________________________ ...
Alkene

In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. Alkene, olefin, and olefine are used often interchangeably (see nomenclature section below). Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a homologous series of hydrocarbons with the general formula CnH2n. Alkenes have two hydrogen atoms less than the corresponding alkane (with the same number of carbon atoms). The simplest alkene, ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene is the organic compound produced on the largest scale industrially. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.