Formation of C-C Bonds via Catalytic Hydrogenation and Transfer
... Abstract: Carbon-carbon bond formation lies at the heart of chemical synthesis. Research in the Krische laboratory reveals reductive C-C bond formation can be achieved under the conditions of catalytic hydrogenation. This concept is extended further via “C-C bond forming transfer hydrogenations”, wh ...
... Abstract: Carbon-carbon bond formation lies at the heart of chemical synthesis. Research in the Krische laboratory reveals reductive C-C bond formation can be achieved under the conditions of catalytic hydrogenation. This concept is extended further via “C-C bond forming transfer hydrogenations”, wh ...
Carbon and the Molecular Diversity of Life
... Organic chemistry is carbon chemistry. Carbon has little tendency to gain or lose electrons. It has a valence number of 4 and forms four covalent bonds. Each carbon atom in a carbon compound is an intersection point and so a molecule can branch off in four directions. This makes it TETRAVALENT. Sing ...
... Organic chemistry is carbon chemistry. Carbon has little tendency to gain or lose electrons. It has a valence number of 4 and forms four covalent bonds. Each carbon atom in a carbon compound is an intersection point and so a molecule can branch off in four directions. This makes it TETRAVALENT. Sing ...
Chemistry 322 Experiment #3 Data Sheet
... 4. Flow Chart. Fill in the empty rectangles with the appropriate cations. In the empty ovals, fill in the appropriate methylpentene structures. H+ OH ...
... 4. Flow Chart. Fill in the empty rectangles with the appropriate cations. In the empty ovals, fill in the appropriate methylpentene structures. H+ OH ...
Document
... • Fuels (e.g. the RM1.92 RM2.10 Ron95) are made up mostly of alkanes. In excess oxygen • CnH2n+2 + (1.5n+½)O2 nCO2 + (n+1)H2O ...
... • Fuels (e.g. the RM1.92 RM2.10 Ron95) are made up mostly of alkanes. In excess oxygen • CnH2n+2 + (1.5n+½)O2 nCO2 + (n+1)H2O ...
Word - chemmybear.com
... Build models of molecules. State the # of atoms and # of elements in a molecule. Draw isomers of molecules and recognize isomers. Know the bond angle of H-C-H bond. State the bonding capacity of C, H, O, Cl, Br, and I. Given the name of a hydrocarbon, write its formula and vice versa. ...
... Build models of molecules. State the # of atoms and # of elements in a molecule. Draw isomers of molecules and recognize isomers. Know the bond angle of H-C-H bond. State the bonding capacity of C, H, O, Cl, Br, and I. Given the name of a hydrocarbon, write its formula and vice versa. ...
Organic Chemistry I: Reactions and Overview
... Most of the solvents with abbreviated names are polar aprotic ...
... Most of the solvents with abbreviated names are polar aprotic ...
Unit 15 Organic Chemistry Notes
... CH4 CH3OH CH3CH2CH2OH Tetrahedral shape-CH4: 4 bonds spread out to the 4 corners ...
... CH4 CH3OH CH3CH2CH2OH Tetrahedral shape-CH4: 4 bonds spread out to the 4 corners ...
Organic/Biological Chemistry
... – As the C-C bond begins to rotate (moving from cis to trans) the ...
... – As the C-C bond begins to rotate (moving from cis to trans) the ...
Addition reactions
... Addition reactions only occur with unsaturated compounds, that is, compounds containing a carbon to carbon double bond or a carbon to carbon triple bond. In other words, alkenes or alkynes. Addition to alkenes ...
... Addition reactions only occur with unsaturated compounds, that is, compounds containing a carbon to carbon double bond or a carbon to carbon triple bond. In other words, alkenes or alkynes. Addition to alkenes ...
organic chemistry i
... Chlorination: a substitution reaction Control of chlorination Reaction with other halogens: halogenation Relative reactivity Reaction mechanisms Mechanism of chlorination. Free radicals Chain reactions Inhibitors Heat of reaction Energy of activation ...
... Chlorination: a substitution reaction Control of chlorination Reaction with other halogens: halogenation Relative reactivity Reaction mechanisms Mechanism of chlorination. Free radicals Chain reactions Inhibitors Heat of reaction Energy of activation ...
organic chem - WordPress.com
... propionic acid and butyric acid; the first four prefixes were named after them, respectively). Prefixes of 11 and higher are much less common, and can be found on Wikipedia easily if you happen to come across them. ALKANES This is the simplest class of organic molecules; they contain only carbon and ...
... propionic acid and butyric acid; the first four prefixes were named after them, respectively). Prefixes of 11 and higher are much less common, and can be found on Wikipedia easily if you happen to come across them. ALKANES This is the simplest class of organic molecules; they contain only carbon and ...
The alkenes
... Many are prepared by a free radical process involving high pressure, high temperature and a catalyst. The catalyst is usually a substance (e.g. an organic peroxide) which readily breaks up to form radicals whichinitiate a chain reaction. Another famous type of catalyst is a Ziegler-Natta catalyst (n ...
... Many are prepared by a free radical process involving high pressure, high temperature and a catalyst. The catalyst is usually a substance (e.g. an organic peroxide) which readily breaks up to form radicals whichinitiate a chain reaction. Another famous type of catalyst is a Ziegler-Natta catalyst (n ...
Chemistry: Spring Semester Lecture Notes - Teach-n-Learn-Chem
... Alkanes: modification for substituent hydrocarbon (HC) groups 1. Number the “longest chain” carbons. Start with the end nearest a branch. 2. Name and give the #ed location of each substituent. -- HC substituent groups use the prefixes, but end in –yl. 3. List substituents in alphabetical order. EX. ...
... Alkanes: modification for substituent hydrocarbon (HC) groups 1. Number the “longest chain” carbons. Start with the end nearest a branch. 2. Name and give the #ed location of each substituent. -- HC substituent groups use the prefixes, but end in –yl. 3. List substituents in alphabetical order. EX. ...
Chapter 18 - Hope Charter School
... placement of the branches can create isomers—these are geometric isomers 3) Geometric isomer names (see page 6310 a) cis- if on the same side b) trans- if on opposite sides d. Reactivity of alkenes 1) more reactive than alkanes, because the extra electrons in the double bond are not held as tightly ...
... placement of the branches can create isomers—these are geometric isomers 3) Geometric isomer names (see page 6310 a) cis- if on the same side b) trans- if on opposite sides d. Reactivity of alkenes 1) more reactive than alkanes, because the extra electrons in the double bond are not held as tightly ...
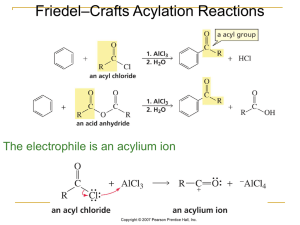
Slide 1
... It is not possible to obtain a good yield of an alkylbenzene containing a straight-chain group via Friedel–Crafts alkylation due to carbocation ...
... It is not possible to obtain a good yield of an alkylbenzene containing a straight-chain group via Friedel–Crafts alkylation due to carbocation ...
Group G
... This article discusses how many people take the use of over the counter (OTC) drugs too lightly. These drugs in high doses or with frequent use can carry certain harmful effects on the body. One example cited is the use of the chemical phenylpropanolamine (PPA) in many OTC drugs. Recently a study at ...
... This article discusses how many people take the use of over the counter (OTC) drugs too lightly. These drugs in high doses or with frequent use can carry certain harmful effects on the body. One example cited is the use of the chemical phenylpropanolamine (PPA) in many OTC drugs. Recently a study at ...
Erik`s Chemistry: Organic Chemistry Notes - ECHS Chemistry
... reaction). Also are involved in substitution reactions. ...
... reaction). Also are involved in substitution reactions. ...
Alkene

In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. Alkene, olefin, and olefine are used often interchangeably (see nomenclature section below). Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a homologous series of hydrocarbons with the general formula CnH2n. Alkenes have two hydrogen atoms less than the corresponding alkane (with the same number of carbon atoms). The simplest alkene, ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene is the organic compound produced on the largest scale industrially. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.