Carbocation Stability
... Tertiary carbocations are more stable than secondary, which are more stable than primary, which are more stable than methyl. Tertiary alcohols react faster than secondary, which react faster than primary, which react faster than methanol. ...
... Tertiary carbocations are more stable than secondary, which are more stable than primary, which are more stable than methyl. Tertiary alcohols react faster than secondary, which react faster than primary, which react faster than methanol. ...
CCCH110D Inorganic vs Organic NOTES
... Alkenes and Alkynes undergo addition reactions in which atoms add across the multiple bonds. For example the addition of chlorine converts the carbon carbon double bond into a single bond because each carbon atom now has a new bond to a chlorine atom. Alkenes and Alkynes can also add hydrogen in hyd ...
... Alkenes and Alkynes undergo addition reactions in which atoms add across the multiple bonds. For example the addition of chlorine converts the carbon carbon double bond into a single bond because each carbon atom now has a new bond to a chlorine atom. Alkenes and Alkynes can also add hydrogen in hyd ...
Basic Chemistry
... Composition of Matter- Elements Isotopes are represented differently, showing their atomic mass. Examples: carbon – 14 is an isotope of carbon with a mass of 14 (the normal mass is 12) iodine – 131 is an isotope of iodine with a mass of 131 (the normal mass is 127) ...
... Composition of Matter- Elements Isotopes are represented differently, showing their atomic mass. Examples: carbon – 14 is an isotope of carbon with a mass of 14 (the normal mass is 12) iodine – 131 is an isotope of iodine with a mass of 131 (the normal mass is 127) ...
MATERIAL FOR GRADE 9 FINAL EXAM 2016
... • Explain why most atoms form chemical bonds. • Differentiate ionic and covalent bonding. • Explain why most chemical bonding is neither purely ionic nor purely covalent. • Classify bonding type according to electronegativity differences. Covalent bonding and molecular compounds • Define molecule an ...
... • Explain why most atoms form chemical bonds. • Differentiate ionic and covalent bonding. • Explain why most chemical bonding is neither purely ionic nor purely covalent. • Classify bonding type according to electronegativity differences. Covalent bonding and molecular compounds • Define molecule an ...
Synthesis of Alum Lab
... 2Al(s) + 2K+(aq) + 2OH-(aq) + 6H2O(l) 2[Al(OH)4]-(aq) + 2K+(aq) + 3H2(g) The oxidation and reduction half reactions: ...
... 2Al(s) + 2K+(aq) + 2OH-(aq) + 6H2O(l) 2[Al(OH)4]-(aq) + 2K+(aq) + 3H2(g) The oxidation and reduction half reactions: ...
Chemical reactions occur with outer level electrons so that is the
... A Na atom has 11+ and 11- IF it loses one electron then 11+ and 10Atoms with more than 4 electrons will gain electrons For Oxygen it is easier to gain 2 electrons than to lose 6 electrons An O atom has 8+ and 8- IF it gains two electrons then 8+ and 10- ...
... A Na atom has 11+ and 11- IF it loses one electron then 11+ and 10Atoms with more than 4 electrons will gain electrons For Oxygen it is easier to gain 2 electrons than to lose 6 electrons An O atom has 8+ and 8- IF it gains two electrons then 8+ and 10- ...
group iv elements
... Carbon exists either as a diamond formation or as graphite, in either form, there are strong covalent bonds between the carbon atoms which must be broken, resulting in a extremely high melting point. Only in graphite is electrical conductivity observed but as expected it is very low. Both silicon an ...
... Carbon exists either as a diamond formation or as graphite, in either form, there are strong covalent bonds between the carbon atoms which must be broken, resulting in a extremely high melting point. Only in graphite is electrical conductivity observed but as expected it is very low. Both silicon an ...
Organic Molecules: Introduction and key concepts
... In organic chemistry, a functional group is a speci c group of atoms within molecules, that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a par ...
... In organic chemistry, a functional group is a speci c group of atoms within molecules, that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a par ...
1.2 The Chemicals of Life - Father Michael McGivney
... 3 carbons....prop 4 carbons....but 5 carbons....pent ...
... 3 carbons....prop 4 carbons....but 5 carbons....pent ...
Chapters 9 and 10
... structure. Indicate whether the molecule is polar or nonpolar, and justify your answer. 13. 2002 #6c. Use the principles of atomic structure and/or chemical bonding to explain each of the following. In each part, your answer must include references to both substances. a. The carbon – to – carbon bon ...
... structure. Indicate whether the molecule is polar or nonpolar, and justify your answer. 13. 2002 #6c. Use the principles of atomic structure and/or chemical bonding to explain each of the following. In each part, your answer must include references to both substances. a. The carbon – to – carbon bon ...
Chemical Formulas and Composition Stoichiometry
... elements combine with one another in small whole-number ratios. • The relative _____ and ____ of atoms are constant in a given compound. ...
... elements combine with one another in small whole-number ratios. • The relative _____ and ____ of atoms are constant in a given compound. ...
Chapter 17: Molecular Modeling Problems
... there is at least one carbonyl group in the molecule. In fact, one of the most common practical uses of infrared spectroscopy is to confirm that a molecule contains carbonyl functionality. Examine the infrared spectrum of acetone (access it from the database accessible from SpartanModel). Is there a ...
... there is at least one carbonyl group in the molecule. In fact, one of the most common practical uses of infrared spectroscopy is to confirm that a molecule contains carbonyl functionality. Examine the infrared spectrum of acetone (access it from the database accessible from SpartanModel). Is there a ...
Name Period_______________ Due
... 7) Fill in the names and structural formulas for the following chemical formulas. Then construct a 3-D model of each of the molecules. When you have completed all of the models, call your teacher so that your work can be checked. Do not disassemble any of the models until your work has been checked. ...
... 7) Fill in the names and structural formulas for the following chemical formulas. Then construct a 3-D model of each of the molecules. When you have completed all of the models, call your teacher so that your work can be checked. Do not disassemble any of the models until your work has been checked. ...
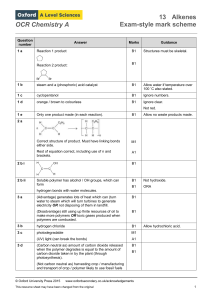
OCR Chemistry A Question number Answer Marks Guidance 1 a
... (In order to have cis or trans isomers) each C atom of the CC double bond must have two different substituent groups and one of those groups must be ...
... (In order to have cis or trans isomers) each C atom of the CC double bond must have two different substituent groups and one of those groups must be ...
Cl -1
... 3. The more-electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. 4. The oxidation number of fluorine in a compound is always -1. 5. Oxygen has an oxidation number of -2 unless it is combined with F (when it is +2), or it is in a per ...
... 3. The more-electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. 4. The oxidation number of fluorine in a compound is always -1. 5. Oxygen has an oxidation number of -2 unless it is combined with F (when it is +2), or it is in a per ...
Document
... • Carbon has the unusual ability of bonding to itself to form long chains or rings of carbon atoms. • Carbon forms strong bonds to other nonmetals such as hydrogen, nitrogen, oxygen, sulfur, and the halogens. • Several million (11 million-plus) are known, and the number continues to grow rapidly. • ...
... • Carbon has the unusual ability of bonding to itself to form long chains or rings of carbon atoms. • Carbon forms strong bonds to other nonmetals such as hydrogen, nitrogen, oxygen, sulfur, and the halogens. • Several million (11 million-plus) are known, and the number continues to grow rapidly. • ...
Honors Midterm - Stamford High School
... don't actually make a list of how many of each atom you have? You won't. You have to make an inventory of how many atoms of each element you have, and then you have to keep it current throughout the whole problem. 4. Write numbers (coefficients) in front of each of the boxes until the inventory for ...
... don't actually make a list of how many of each atom you have? You won't. You have to make an inventory of how many atoms of each element you have, and then you have to keep it current throughout the whole problem. 4. Write numbers (coefficients) in front of each of the boxes until the inventory for ...
organic chemistry 307
... dots and 6 dots. Each different pattern corresponds to a different carbon skeleton, which is the starting point for describing and naming all organic compounds. Note it’s the pattern of attachment that counts, how many dots a given dot is connected to, not how we draw it, straight, zig-zag, or bent. ...
... dots and 6 dots. Each different pattern corresponds to a different carbon skeleton, which is the starting point for describing and naming all organic compounds. Note it’s the pattern of attachment that counts, how many dots a given dot is connected to, not how we draw it, straight, zig-zag, or bent. ...
CHEM 2414
... 1. For a monosubstituted cycloalkane the ring supplies the root name (table above) and the substituent group is named as usual. A location number is unnecessary. 2. If the alkyl substituent is large and/or complex, the ring may be named as a substituent group on an alkane. 3. If two different substi ...
... 1. For a monosubstituted cycloalkane the ring supplies the root name (table above) and the substituent group is named as usual. A location number is unnecessary. 2. If the alkyl substituent is large and/or complex, the ring may be named as a substituent group on an alkane. 3. If two different substi ...
Homoaromaticity

Homoaromaticity in organic chemistry refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.The concept of homoaromaticity was pioneered by Saul Winstein in 1959, prompted by his studies of the “tris-homocyclopropenyl” cation. Since the publication of Winstein's paper, much research has been devoted to understanding and classifying these molecules, which represent an additional “class” of aromatic molecules included under the continuously broadening definition of aromaticity. To date, homoaromatic compounds are known to exist as cationic and anionic species, and some studies support the existence of neutral homoaromatic molecules, though these are less common. The 'homotropylium' cation (C8H9+) is perhaps the best studied example of a homoaromatic compound.