1 - contentextra
... Aldehydes (alkanals) A homologous series of compounds with the general formula RCHO, where the –CHO group (the aldehyde group) consists of a carbonyl group attached to a hydrogen atom. R is an alkyl or aryl group. Alkyl group A group, with the general formula CnH2n + 1, obtained by removing a hydrog ...
... Aldehydes (alkanals) A homologous series of compounds with the general formula RCHO, where the –CHO group (the aldehyde group) consists of a carbonyl group attached to a hydrogen atom. R is an alkyl or aryl group. Alkyl group A group, with the general formula CnH2n + 1, obtained by removing a hydrog ...
Chem 30A Fa_06 FE Review
... Which of the following mixtures will form a buffer solution? (a) a solution containing 1 M HCl and 1 M NaCl. (b) a solution containing 1 M HCl and 1 M NaOH (c) a solution containing 1 M NH4NO3 and 1 M NH4OH (d) a solution containing 1 M NH4NO3 and 1 M NaNO3 ...
... Which of the following mixtures will form a buffer solution? (a) a solution containing 1 M HCl and 1 M NaCl. (b) a solution containing 1 M HCl and 1 M NaOH (c) a solution containing 1 M NH4NO3 and 1 M NH4OH (d) a solution containing 1 M NH4NO3 and 1 M NaNO3 ...
Ch03_ Lecture
... (from the side of higher concentration of the ion to a side of lower ion concentration). ...
... (from the side of higher concentration of the ion to a side of lower ion concentration). ...
Chapter 4: Carbon
... Carbon forms the backbone of proteins, fats, carbohydrates, and nucleic acids We all eat at the SPONCH CaFé (but not in that order…HOCNCaPSFe is just too hard to pronounce!!) Percentages don’t vary much from one organism to another. However, because of carbon’s versatility, these few elements can be ...
... Carbon forms the backbone of proteins, fats, carbohydrates, and nucleic acids We all eat at the SPONCH CaFé (but not in that order…HOCNCaPSFe is just too hard to pronounce!!) Percentages don’t vary much from one organism to another. However, because of carbon’s versatility, these few elements can be ...
Chapter 6 Chemical Bonding
... atomic symbols and numeric subscripts Ex: Na17Cl17 A Molecular formula shows the types and numbers of atoms combined in a single molecule of a molecular compound Ex: H2O; C2H6 ...
... atomic symbols and numeric subscripts Ex: Na17Cl17 A Molecular formula shows the types and numbers of atoms combined in a single molecule of a molecular compound Ex: H2O; C2H6 ...
Carbon and the Molecular Diversity of Life
... of same elements but different structures and different properties ...
... of same elements but different structures and different properties ...
Organic Synthesis of aromatic compounds
... • Explain that synthetic molecules often contain a mixture of optical isomers, whereas natural molecules often have only one optical isomer. • Explain that the synthesis of a pharmaceutical that is a single optical isomer increases costs, reduces side effects and improves pharmacological activity. • ...
... • Explain that synthetic molecules often contain a mixture of optical isomers, whereas natural molecules often have only one optical isomer. • Explain that the synthesis of a pharmaceutical that is a single optical isomer increases costs, reduces side effects and improves pharmacological activity. • ...
An Introduction to Chemistry
... be seen with the naked eye but is large enough to be seen with an optical microscope is considered to be microscopic. • MACROSCOPIC: Anything that is large enough to be seen with the naked eye is considered to be macroscopic. ...
... be seen with the naked eye but is large enough to be seen with an optical microscope is considered to be microscopic. • MACROSCOPIC: Anything that is large enough to be seen with the naked eye is considered to be macroscopic. ...
Day 8
... You have probably noticed that the names changed slightly for hydrocarbons with double bonds. This series is called the alkenes. How can you tell from their names? Hydrocarbons with triple bonds are called alkynes. A four carbon compound with a triple bond is called butyne. 9. Here’s a challenge for ...
... You have probably noticed that the names changed slightly for hydrocarbons with double bonds. This series is called the alkenes. How can you tell from their names? Hydrocarbons with triple bonds are called alkynes. A four carbon compound with a triple bond is called butyne. 9. Here’s a challenge for ...
Cyclopentane (C5H10) Nomenclature of cycloalcanes Saturated
... To name these compounds, the root name is taken from the total number of carbons in both rings. Thus for example I above, there are 9 (nine) carbons in both rings together leading to the name spirononane. However, the size of the two rings may vary, for if you look closely at Examples II and III ab ...
... To name these compounds, the root name is taken from the total number of carbons in both rings. Thus for example I above, there are 9 (nine) carbons in both rings together leading to the name spirononane. However, the size of the two rings may vary, for if you look closely at Examples II and III ab ...
Module 8: Descriptive Chemistry
... – Water-gas reaction: C(s) + H2O(g) à CO(g) + H2(g) – Water-gas shift reaction: CO(g) + H2O(g) à CO2(g) + H2(g) – Reforming of methane (but in principle any hydrocarbon): CH4(g) + H2O(g) à CO(g) + 3H2(g) • Principal commercial source of hydrogen ...
... – Water-gas reaction: C(s) + H2O(g) à CO(g) + H2(g) – Water-gas shift reaction: CO(g) + H2O(g) à CO2(g) + H2(g) – Reforming of methane (but in principle any hydrocarbon): CH4(g) + H2O(g) à CO(g) + 3H2(g) • Principal commercial source of hydrogen ...
Valence Electrons
... Valence electrons are electrons in the _outer shell_ of an atom. Nitrogen has _five_ valence electrons. Carbon has _four_ valence electrons. Fluorine has _seven_ valence electrons. What is the name of the group of elements that have 8 valence electrons? Noble gases ...
... Valence electrons are electrons in the _outer shell_ of an atom. Nitrogen has _five_ valence electrons. Carbon has _four_ valence electrons. Fluorine has _seven_ valence electrons. What is the name of the group of elements that have 8 valence electrons? Noble gases ...
Carbon, because of its valence electrons, can form four bonds and
... Hydrocarbons are the simplest group of organic compounds. These compounds are groups by the types of bonding between carbon atoms (single, double, or triple) Saturated Hydrocarbons—hydrocarbons in which each carbon atom in the molecule forms four single covalent bonds with other atoms Alkanes—hydroc ...
... Hydrocarbons are the simplest group of organic compounds. These compounds are groups by the types of bonding between carbon atoms (single, double, or triple) Saturated Hydrocarbons—hydrocarbons in which each carbon atom in the molecule forms four single covalent bonds with other atoms Alkanes—hydroc ...
Long-Range Coupling
... H’s on aromatic rings may couple with non-neighboring protons due to long-range coupling. You will see this in lab! Why? Nuclei “communicate” via bonding electrons - p electrons that are in resonance will allow non-neighboring H’s to “communicate” and couple/split. This leads to complex splitting. ...
... H’s on aromatic rings may couple with non-neighboring protons due to long-range coupling. You will see this in lab! Why? Nuclei “communicate” via bonding electrons - p electrons that are in resonance will allow non-neighboring H’s to “communicate” and couple/split. This leads to complex splitting. ...
Document
... c. 3.3 Dalton’s Atomic Theory i. In the early 1800’s English scientist John Dalton came up with an explanation of how atoms combine to form compounds. ii. Dalton’s atomic theory has five main points: 1. Elements are made up of atoms. 2. Each atom of an element is exactly the same as all the others. ...
... c. 3.3 Dalton’s Atomic Theory i. In the early 1800’s English scientist John Dalton came up with an explanation of how atoms combine to form compounds. ii. Dalton’s atomic theory has five main points: 1. Elements are made up of atoms. 2. Each atom of an element is exactly the same as all the others. ...
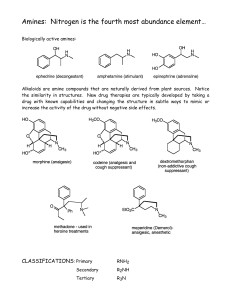
Amines: Nitrogen is the fourth most abundance element…
... naphthalene is a neutral organic compound without any acidic nor basic functionality. Benzoic acid has an acidic proton that reacts with bases. Pyridine is a base that reacts with acids. Let’s separate them. Add aqueous acid to the three compounds. What happens to each? Nothing happens to naphthalen ...
... naphthalene is a neutral organic compound without any acidic nor basic functionality. Benzoic acid has an acidic proton that reacts with bases. Pyridine is a base that reacts with acids. Let’s separate them. Add aqueous acid to the three compounds. What happens to each? Nothing happens to naphthalen ...
CHEMISTRY
... Matter that can not be broken down into simpler substances under normal lab conditions Contains only one kind of atom ...
... Matter that can not be broken down into simpler substances under normal lab conditions Contains only one kind of atom ...
Chapter 17 Allylic and Benzylic Reactivity
... As in electrophilic substitution, the resonance effect of the p-methoxy group strongly outweighs its electronwithdrawing polar effect. In compound (3), there is a similar resonance effect; however, the polar effects of halogen substituents outweigh their resonance effects. Consequently, compound (3) ...
... As in electrophilic substitution, the resonance effect of the p-methoxy group strongly outweighs its electronwithdrawing polar effect. In compound (3), there is a similar resonance effect; however, the polar effects of halogen substituents outweigh their resonance effects. Consequently, compound (3) ...
CHAPTER 4 CARBON AND THE MOLECULAR
... When two carbon atoms are joined by a double bond, all bonds around the carbons are in the same plane. They have a flat, three-dimensional structure. The electron configuration of carbon gives it compatibility to form covalent bonds with many different elements. The valences of carbon and it ...
... When two carbon atoms are joined by a double bond, all bonds around the carbons are in the same plane. They have a flat, three-dimensional structure. The electron configuration of carbon gives it compatibility to form covalent bonds with many different elements. The valences of carbon and it ...
Survival Organic Chemistry Molecular Models The goal in this
... out that you do not make a conformer, which is a different special arrangement of a compound but contains the same arrangement of carboncarbon bonds. Use your sheet of paper to draw the Lewis structure for each of the compounds and any structural isomers they may have. 3. Draw 4 structural isomers o ...
... out that you do not make a conformer, which is a different special arrangement of a compound but contains the same arrangement of carboncarbon bonds. Use your sheet of paper to draw the Lewis structure for each of the compounds and any structural isomers they may have. 3. Draw 4 structural isomers o ...
Carbon and the Molecular Diversity of Life
... Organic Molecules and the Origin of Life on Earth • Stanley Miller’s classic experiment demonstrated the abiotic synthesis of organic compounds • Experiments support the idea that abiotic synthesis of organic compounds, perhaps near volcanoes, could have been a stage in the origin of life ...
... Organic Molecules and the Origin of Life on Earth • Stanley Miller’s classic experiment demonstrated the abiotic synthesis of organic compounds • Experiments support the idea that abiotic synthesis of organic compounds, perhaps near volcanoes, could have been a stage in the origin of life ...
Homoaromaticity

Homoaromaticity in organic chemistry refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.The concept of homoaromaticity was pioneered by Saul Winstein in 1959, prompted by his studies of the “tris-homocyclopropenyl” cation. Since the publication of Winstein's paper, much research has been devoted to understanding and classifying these molecules, which represent an additional “class” of aromatic molecules included under the continuously broadening definition of aromaticity. To date, homoaromatic compounds are known to exist as cationic and anionic species, and some studies support the existence of neutral homoaromatic molecules, though these are less common. The 'homotropylium' cation (C8H9+) is perhaps the best studied example of a homoaromatic compound.