1 Organic Compounds – Functional Groups and Physical Properties
... The Nature of Carbon – Carbon Multiple Bonds: In a carbon-carbon multiple bond, either a double bond or a triple bond, there are two types of bonds present. These bonding types are known as sigma bonds or pi bonds. There are three key concepts about chemical bonding: (1) Different types of atomic or ...
... The Nature of Carbon – Carbon Multiple Bonds: In a carbon-carbon multiple bond, either a double bond or a triple bond, there are two types of bonds present. These bonding types are known as sigma bonds or pi bonds. There are three key concepts about chemical bonding: (1) Different types of atomic or ...
Chapter 4
... substances prepared directly from pure elements. In 1953, Stanley Miller at the University of Chicago set up a laboratory simulation of possible chemical conditions on the primitive Earth and demonstrated the spontaneous synthesis of organic compounds. o The mixture of gases Miller created probably ...
... substances prepared directly from pure elements. In 1953, Stanley Miller at the University of Chicago set up a laboratory simulation of possible chemical conditions on the primitive Earth and demonstrated the spontaneous synthesis of organic compounds. o The mixture of gases Miller created probably ...
CHAPTER 4 CARBON AND THE MOLECULAR DIVERSITY OF LIFE
... These chemical groups may be involved in chemical reactions or may contribute to the shape and function of the organic molecule in a characteristic way, giving it unique properties. o ...
... These chemical groups may be involved in chemical reactions or may contribute to the shape and function of the organic molecule in a characteristic way, giving it unique properties. o ...
Chemistry to Remember
... (the dissolving medium). The solute is dispersed into molecules or ions consistently throughout the solution. Any given unit volume of solution will have an equal amount of solute molecules or ions. The amount of solute per unit volume of solvent is the concentration of the solution and can be expre ...
... (the dissolving medium). The solute is dispersed into molecules or ions consistently throughout the solution. Any given unit volume of solution will have an equal amount of solute molecules or ions. The amount of solute per unit volume of solvent is the concentration of the solution and can be expre ...
Alkenes Group
... undergo incomplete combustion. • Finally, alkene molecules can add on to each other to form compounds with very long carbon chains. These compounds are called polymers C2H4 - -(CH2—CH2)-n ...
... undergo incomplete combustion. • Finally, alkene molecules can add on to each other to form compounds with very long carbon chains. These compounds are called polymers C2H4 - -(CH2—CH2)-n ...
Organic Compounds
... Models are often used by chemists to visualize molecular structures. Structural differences between functional groups provide reasons for differences in chemical reactivity. In preparation for this laboratory exercise, review the sections in your text book pertaining to molecular structure of compou ...
... Models are often used by chemists to visualize molecular structures. Structural differences between functional groups provide reasons for differences in chemical reactivity. In preparation for this laboratory exercise, review the sections in your text book pertaining to molecular structure of compou ...
Honors Biology Chapter 2 Power Point
... • What three possible atoms can make a hydrogen bond with hydrogen? • List the forces in order of strength. ...
... • What three possible atoms can make a hydrogen bond with hydrogen? • List the forces in order of strength. ...
Chemistry
... 4. Reduction of Amides and Schmidt reaction. Physical properties and basic character Comparative basic strength of Ammonia, methyl amine, dimethyl amine, trimethyl amine and aniline - comparative basic strength of aniline, N- methylaniline and N,N-dimethyl aniline (in aqueous and non-aqueous medium) ...
... 4. Reduction of Amides and Schmidt reaction. Physical properties and basic character Comparative basic strength of Ammonia, methyl amine, dimethyl amine, trimethyl amine and aniline - comparative basic strength of aniline, N- methylaniline and N,N-dimethyl aniline (in aqueous and non-aqueous medium) ...
Nucleophilic Addition to Carbonyl Groups
... Aldehydes and ketones that have a C=O bond , but no O-H bond, cannot form hydrogen bonds with one another, as alcohols. Aldehyde and ketones therefore have relatively higher boiling points than hydrocarbons, but less than alcohols. Low molecular weight aldehydes and ketones are water soluble as they ...
... Aldehydes and ketones that have a C=O bond , but no O-H bond, cannot form hydrogen bonds with one another, as alcohols. Aldehyde and ketones therefore have relatively higher boiling points than hydrocarbons, but less than alcohols. Low molecular weight aldehydes and ketones are water soluble as they ...
Main Group Organometallic Compounds
... • Reaction of SiCl4 with organolithium, Grignard reagents or organoaluminum compounds • Hydrosilation of alkenes • Direct reaction of RX with Si in the presence of a Cu ...
... • Reaction of SiCl4 with organolithium, Grignard reagents or organoaluminum compounds • Hydrosilation of alkenes • Direct reaction of RX with Si in the presence of a Cu ...
Chemical Bonding I
... summing the valence electrons of each atom. (Be sure to take ions into account!) 3) Distribute the electrons among the atoms giving octets to all atoms other than H (duet for it). 4) If any a ...
... summing the valence electrons of each atom. (Be sure to take ions into account!) 3) Distribute the electrons among the atoms giving octets to all atoms other than H (duet for it). 4) If any a ...
The Formation of Comets
... The life-giving ideas of chemistry are not reducible to physics. Or, if one tries to reduce them, they wilt at the edges, lose not only much of their meaning, but interest too. And, most importantly, they lose their chemical utility—their ability to relate seemingly disparate compounds to each other ...
... The life-giving ideas of chemistry are not reducible to physics. Or, if one tries to reduce them, they wilt at the edges, lose not only much of their meaning, but interest too. And, most importantly, they lose their chemical utility—their ability to relate seemingly disparate compounds to each other ...
Problem Set 3_Chem165_Sp2014
... compounds is volatilized (evaporated into the gas phase) into an inert gas stream that is flowing down a long thin tube packed with material. The different compounds in the mixture move at different rates down the tube, so they are separated by the time they reach the end of the tube (see: http://en ...
... compounds is volatilized (evaporated into the gas phase) into an inert gas stream that is flowing down a long thin tube packed with material. The different compounds in the mixture move at different rates down the tube, so they are separated by the time they reach the end of the tube (see: http://en ...
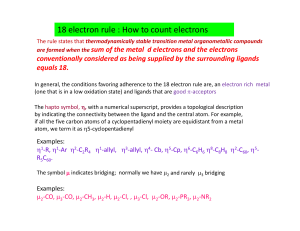
18 electron rule : How to count electrons
... In general, the conditions favoring adherence to the 18 electron rule are, an electron rich metal (one that is in a low oxidation state) and ligands that are good π‐acceptors The hapto symbol, η, with a numerical superscript, provides a topological description by indicating the connectivity betw ...
... In general, the conditions favoring adherence to the 18 electron rule are, an electron rich metal (one that is in a low oxidation state) and ligands that are good π‐acceptors The hapto symbol, η, with a numerical superscript, provides a topological description by indicating the connectivity betw ...
Chapter 10
... The coefficients of a balanced equation relate the moles (numbers) of any compound to the moles (numbers) of any other compound in the equation. These molar ratios are used to 'convert' between any two compounds, whether they are reactants or products. This allows us to calculate moles of reac ...
... The coefficients of a balanced equation relate the moles (numbers) of any compound to the moles (numbers) of any other compound in the equation. These molar ratios are used to 'convert' between any two compounds, whether they are reactants or products. This allows us to calculate moles of reac ...
OrganicChemistrySV
... - named by changing the alkane ending of –e to –amine and then numbering the alkane chain to show the location of the amine group ...
... - named by changing the alkane ending of –e to –amine and then numbering the alkane chain to show the location of the amine group ...
Organic Chemistry = ______________________ ________________________
... - named by changing the alkane ending of –e to –amine and then numbering the alkane chain to show the location of the amine group ...
... - named by changing the alkane ending of –e to –amine and then numbering the alkane chain to show the location of the amine group ...
Topic 10: Organic Chemistry P1: …….. / 15p. P2: ……. / 29p.
... Deduce the empirical formula of methyl 2-hydroxy benzoate and draw the full structural formula, including any multiple bonds that may be present. The computer-generated representation shown does not distinguish between single and ...
... Deduce the empirical formula of methyl 2-hydroxy benzoate and draw the full structural formula, including any multiple bonds that may be present. The computer-generated representation shown does not distinguish between single and ...
notes
... Carbon chemistry is that of COVALENT BONDING. Usually nonpolar. Single bond Double bond C-C, C-N, C-S, C-O….? S-S Because S is the closest in chemical structure to C its possible their would be unique compounds with sulfur and in areas with lots of sulfur (ocean vents) their would be S-S life forms ...
... Carbon chemistry is that of COVALENT BONDING. Usually nonpolar. Single bond Double bond C-C, C-N, C-S, C-O….? S-S Because S is the closest in chemical structure to C its possible their would be unique compounds with sulfur and in areas with lots of sulfur (ocean vents) their would be S-S life forms ...
Conformations
... energy, Ea, which is nearly equal to ΔG , the free energy needed to reach the transition state. For ethane, Ea = 2.9 kcal/mol. This barrier is due to torsional strain, which is orbital repulsion (debate continues on this point) caused by eclipsing hydrogens. This energy barrier is called free rotati ...
... energy, Ea, which is nearly equal to ΔG , the free energy needed to reach the transition state. For ethane, Ea = 2.9 kcal/mol. This barrier is due to torsional strain, which is orbital repulsion (debate continues on this point) caused by eclipsing hydrogens. This energy barrier is called free rotati ...
Homoaromaticity

Homoaromaticity in organic chemistry refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.The concept of homoaromaticity was pioneered by Saul Winstein in 1959, prompted by his studies of the “tris-homocyclopropenyl” cation. Since the publication of Winstein's paper, much research has been devoted to understanding and classifying these molecules, which represent an additional “class” of aromatic molecules included under the continuously broadening definition of aromaticity. To date, homoaromatic compounds are known to exist as cationic and anionic species, and some studies support the existence of neutral homoaromatic molecules, though these are less common. The 'homotropylium' cation (C8H9+) is perhaps the best studied example of a homoaromatic compound.