CHM 101
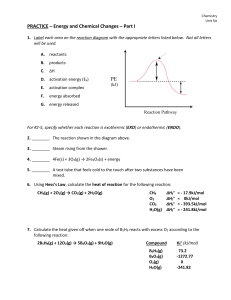
... The reactants in a chemical change have 487 kJ of energy. The change they undergo has a H = -157 kJ. The activation energy for the reaction is 570 kJ. a. Draw the energy vs reaction progress graph on the axes above paying attention to all values. Label a point that represents all products and one t ...
... The reactants in a chemical change have 487 kJ of energy. The change they undergo has a H = -157 kJ. The activation energy for the reaction is 570 kJ. a. Draw the energy vs reaction progress graph on the axes above paying attention to all values. Label a point that represents all products and one t ...
Syllabus
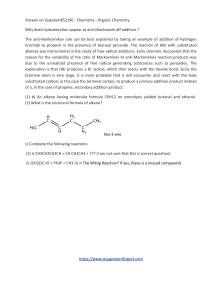
... There will be 12 homework assignments, a midterm and a final. All are given on a take-home basis. If you have any question about a problem before starting to work, you are strongly encouraged to discuss the matter with the professor or fellow students. That is, students are encouraged to discuss and ...
... There will be 12 homework assignments, a midterm and a final. All are given on a take-home basis. If you have any question about a problem before starting to work, you are strongly encouraged to discuss the matter with the professor or fellow students. That is, students are encouraged to discuss and ...
Exothermic vs Endothermic
... Gray's students placed an antacid tablet in a zip lock bag. They recorded the mass of the tablet, 25 grams, and the bag, 60 grams. Then they carefully added 50 grams of water and quickly sealed the bag. The tablet began to fizz and soon disappeared. The bag was filled with gas. If the total mass of ...
... Gray's students placed an antacid tablet in a zip lock bag. They recorded the mass of the tablet, 25 grams, and the bag, 60 grams. Then they carefully added 50 grams of water and quickly sealed the bag. The tablet began to fizz and soon disappeared. The bag was filled with gas. If the total mass of ...
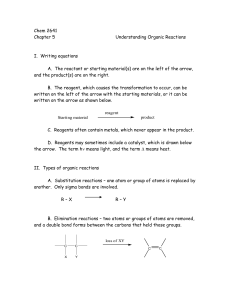
Chem 2641 Chapter 5 Understanding Organic Reactions I. Writing
... The initiation stage – Cl2 reacts with the uv light to form Cl. Propagation stage – The Cl. reacts with CH4 to form CH3. and HCl The CH3. reacts with Cl2 to form CH3Cl and Cl. Termination stage – any two radicals can combine to form a stable product. ...
... The initiation stage – Cl2 reacts with the uv light to form Cl. Propagation stage – The Cl. reacts with CH4 to form CH3. and HCl The CH3. reacts with Cl2 to form CH3Cl and Cl. Termination stage – any two radicals can combine to form a stable product. ...
CH 6
... • By definition, its structure is not stable enough to exist for one vibration • But the structure controls the rate of reaction • So we need to be able to guess about its properties in an informed way • We classify them in general ways and look for trends in reactivity – the conclusions are in the ...
... • By definition, its structure is not stable enough to exist for one vibration • But the structure controls the rate of reaction • So we need to be able to guess about its properties in an informed way • We classify them in general ways and look for trends in reactivity – the conclusions are in the ...
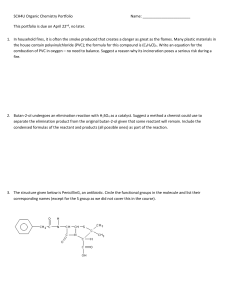
SCH4U Organic Chemistry Portfolio Name: This portfolio is due on
... 5. Nomex is a polymer used to make flame-resistant clothing for firefighters. A portion of its structure is provided below. Write a polymerization reaction showing its production from monomers. What type of reaction is this? ...
... 5. Nomex is a polymer used to make flame-resistant clothing for firefighters. A portion of its structure is provided below. Write a polymerization reaction showing its production from monomers. What type of reaction is this? ...
Assignment 2 Group A and B
... 9) Which of the following alcohols can be prepared by the reaction of methyl formate with excess Grignard reagent? A) 1-pentanol B) 2-pentanol C) 3-pentanol D) 2-methyl-2-pentanol E) 3-methyl-3-pentanol 10) What reagent(s) would you use to accomplish the following conversion? ...
... 9) Which of the following alcohols can be prepared by the reaction of methyl formate with excess Grignard reagent? A) 1-pentanol B) 2-pentanol C) 3-pentanol D) 2-methyl-2-pentanol E) 3-methyl-3-pentanol 10) What reagent(s) would you use to accomplish the following conversion? ...
PowerPoint Presentation - No Slide Title
... Mechanistic path of a reaction: how reactants form products. ...
... Mechanistic path of a reaction: how reactants form products. ...