The Nature of Chemical Reactions
... The formation of carbon dioxide and water from isooctane and oxygen produces the energy used to power engines! As the bonds of isooctane break down, energy is released as the new compounds are formed ...
... The formation of carbon dioxide and water from isooctane and oxygen produces the energy used to power engines! As the bonds of isooctane break down, energy is released as the new compounds are formed ...
II. BIOPHYSICAL CHEMISTRY*
... important reactions, primarily through the use of kinetic methods. ...
... important reactions, primarily through the use of kinetic methods. ...
Aim # 8: How do we write and balance a chemical equation?
... fact that mass has been conserved in this chemical reaction? Pb(NO3)2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq) ...
... fact that mass has been conserved in this chemical reaction? Pb(NO3)2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq) ...
N H CCl3 C O N CCl3 C Cl (ii) SOCl2 7.55 g 7.78 g CCl C N NH N H
... chain of arginine in its conjugate acid form. What is the approximate pKa value for the conjugate acid form of the arginine side chain? (Hint: a nitrogen atom which is part of a double bond, including aromatic N atoms such as in pyridine, are more basic than nitrogen atoms which have only single bon ...
... chain of arginine in its conjugate acid form. What is the approximate pKa value for the conjugate acid form of the arginine side chain? (Hint: a nitrogen atom which is part of a double bond, including aromatic N atoms such as in pyridine, are more basic than nitrogen atoms which have only single bon ...
A.P. Chemistry Complexation Reactions
... Two compounds switch partners and form two new compounds. ...
... Two compounds switch partners and form two new compounds. ...
General Chemistry (II) Chapter 1: Chemical Kinetic 1
... General Chemistry (II) Chapter 1: Chemical Kinetic ...
... General Chemistry (II) Chapter 1: Chemical Kinetic ...
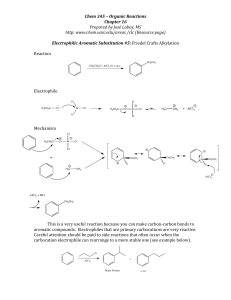
CHEM 2411 – Organic Chemistry I Radicals/Radical Reactions 1
... 9) Draw the major product(s) of the following reaction. Is the product optically active? Explain. ...
... 9) Draw the major product(s) of the following reaction. Is the product optically active? Explain. ...
CHM 111: General Physical Chemistry 3 Units
... solids types of solids and their properties, ionic solids and lattice energy, crystalline solids. Chemical Energetic: definition of some thermodynamic terms, heat, work, internal energy, enthalpy, pressure-volume work. Relationship between internal energy and enthalpy. First law of thermodynamics an ...
... solids types of solids and their properties, ionic solids and lattice energy, crystalline solids. Chemical Energetic: definition of some thermodynamic terms, heat, work, internal energy, enthalpy, pressure-volume work. Relationship between internal energy and enthalpy. First law of thermodynamics an ...