Slide 1 - Mrs. Reed Science Classes
... After calculating the amount of reactant B required to completely react with A, then comparing that amount with the amount of B available, one can determine the a. limiting reactant. b. rate of the reaction. c. energy released in the reaction. d. pathway of the reaction. ...
... After calculating the amount of reactant B required to completely react with A, then comparing that amount with the amount of B available, one can determine the a. limiting reactant. b. rate of the reaction. c. energy released in the reaction. d. pathway of the reaction. ...
Test: "Chemical Equations" (General Chemistry)
... General Chemistry: Chapter 7 Test 1. In endothermic reactions: a. energy is released b. products have less energy than reactants ...
... General Chemistry: Chapter 7 Test 1. In endothermic reactions: a. energy is released b. products have less energy than reactants ...
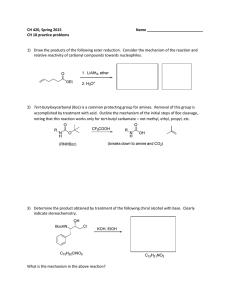
CH 420, Spring 2015 Name ___________________________ CH 18 practice problems
... noting that this reaction works only for tert-butyl carbamate – not methyl, ethyl, propyl, etc. ...
... noting that this reaction works only for tert-butyl carbamate – not methyl, ethyl, propyl, etc. ...
Evaporation - CMA
... and energy is released as new bonds form in products. The net result of these steps depends on the relative sizes of the energies associated with breaking and forming bonds and determines if the reaction absorbs or releases energy The amount of heat involved in a reaction depends not only on what th ...
... and energy is released as new bonds form in products. The net result of these steps depends on the relative sizes of the energies associated with breaking and forming bonds and determines if the reaction absorbs or releases energy The amount of heat involved in a reaction depends not only on what th ...
classification of chemical reactions
... change in matter that produces new substances Example: rusting of iron burning of wood Physical change a change that does not produce a new substance a change in appearance or state Example: chopping wood melting ice Evidence of chemical reactions ...
... change in matter that produces new substances Example: rusting of iron burning of wood Physical change a change that does not produce a new substance a change in appearance or state Example: chopping wood melting ice Evidence of chemical reactions ...
CHAPTER 8
... CHGN122 – Principles of Chemistry II (I,II)- Continuation of CHGN121 Study of matter and energy concentrating on chemical kinetics, thermodynamics, electrochemistry, organic nomenclature, and chemical equilibrium (acid- base, solubility, complexa- tion, and redox). Laboratory experiments emphasizing ...
... CHGN122 – Principles of Chemistry II (I,II)- Continuation of CHGN121 Study of matter and energy concentrating on chemical kinetics, thermodynamics, electrochemistry, organic nomenclature, and chemical equilibrium (acid- base, solubility, complexa- tion, and redox). Laboratory experiments emphasizing ...