m ι
... Schrödinger (wave) equation: • An acceptable solution to Schrödinger equation that states the location of an electron at a given point in space and each wave function is associated with a particular energy E. • A plot of distance versus 2 represents an orbital, a probability distribution map of a ...
... Schrödinger (wave) equation: • An acceptable solution to Schrödinger equation that states the location of an electron at a given point in space and each wave function is associated with a particular energy E. • A plot of distance versus 2 represents an orbital, a probability distribution map of a ...
Lecture 2 - Richard Grotjahn
... Heat – some basic concepts part 2 • Heat capacity is amount of energy to raise temperature of object. Different objects need different amounts of heat to raise their temperature a given amount. • Heat felt or measured is “sensible” heat • Heat used to change the state of an object is “latent” heat. ...
... Heat – some basic concepts part 2 • Heat capacity is amount of energy to raise temperature of object. Different objects need different amounts of heat to raise their temperature a given amount. • Heat felt or measured is “sensible” heat • Heat used to change the state of an object is “latent” heat. ...
Section 15
... product(s). Molecular formulas are usually used to represent the reactants and products and the phase of each substance is sometimes represented by an s, g, or l for solid, gas or liquid; materials that are dissolved in water are designated as aq for aqueous. Coefficients are also placed in front of ...
... product(s). Molecular formulas are usually used to represent the reactants and products and the phase of each substance is sometimes represented by an s, g, or l for solid, gas or liquid; materials that are dissolved in water are designated as aq for aqueous. Coefficients are also placed in front of ...
Chapter2 The First Law of Thermodynamics
... Law of Thermodynamics 3-1-1 Conservation of energy principle Energy can be neither created nor destroyed;it can only change forms 3-1-2 The First Law of Thermodynamics Neither heat nor work can be destroyed;they can only change from one to another, that is: ...
... Law of Thermodynamics 3-1-1 Conservation of energy principle Energy can be neither created nor destroyed;it can only change forms 3-1-2 The First Law of Thermodynamics Neither heat nor work can be destroyed;they can only change from one to another, that is: ...
Atoms and quantum phenomena
... • whatever happens in between emission and absorption, light is always emitted and absorbed in discrete amounts of energy. ...
... • whatever happens in between emission and absorption, light is always emitted and absorbed in discrete amounts of energy. ...
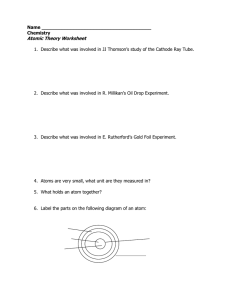
Atomic Theory Worksheet
... 1. Describe what was involved in JJ Thomson’s study of the Cathode Ray Tube. ...
... 1. Describe what was involved in JJ Thomson’s study of the Cathode Ray Tube. ...
UNIT-4-free electron theory
... The resistivity of copper at 200 C is 1.69x10-8 ohm-m and density of free electrons, n=8.5 x 1028m3. Thus The experimentally observed value for mean free path is ten times the above value. Classical theory failed to explain the large values for mean free path. (iv) Relation between electrical & Ther ...
... The resistivity of copper at 200 C is 1.69x10-8 ohm-m and density of free electrons, n=8.5 x 1028m3. Thus The experimentally observed value for mean free path is ten times the above value. Classical theory failed to explain the large values for mean free path. (iv) Relation between electrical & Ther ...
CHAPTER 9: Statistical Physics
... Where 1/8 comes from the restriction to positive values of ni and 2 comes from the fact that there are two possible photon polarizations. Energy is proportional to r, ...
... Where 1/8 comes from the restriction to positive values of ni and 2 comes from the fact that there are two possible photon polarizations. Energy is proportional to r, ...
LECTURE 17 GENERATION/RECOMBINATION Generation of
... ∴ light absorption is relatively weak. Note: all semiconductors are effectively - transparent for light with hv < EG (no possible transition). - absorb for hv > EG (transitions allowed) Recombination of Electrons and Holes Recombination: The movement of an electron from the conduction band to the v ...
... ∴ light absorption is relatively weak. Note: all semiconductors are effectively - transparent for light with hv < EG (no possible transition). - absorb for hv > EG (transitions allowed) Recombination of Electrons and Holes Recombination: The movement of an electron from the conduction band to the v ...
Summary - Clarkson University
... ( E2 + PV2 ) − ( E1 + PV1 ) = Q p Since E , P, and V are state variables , ( E + PV ) must also be a state variable. D efine Enthalpy ( H ) : H ≡ U + PV Assuming negliginle change in potential and kinetic energy we can write H 2 − H1 = Q p ∆H = Q p The enthalpy change of the system is equal to the h ...
... ( E2 + PV2 ) − ( E1 + PV1 ) = Q p Since E , P, and V are state variables , ( E + PV ) must also be a state variable. D efine Enthalpy ( H ) : H ≡ U + PV Assuming negliginle change in potential and kinetic energy we can write H 2 − H1 = Q p ∆H = Q p The enthalpy change of the system is equal to the h ...
STATE UNIVERSITY OF NEW YORK COLLEGE OF TECHNOLOGY CANTON, NEW YORK
... VIII. Models of Electrons in Solids, and Energy Bands in solids A. Models will be used to show how electronic structure emerges from the fundamental interactions of electrons in materials, as described by quantum mechanics. B. Conduction electrons. C. The free-electron gas. D. Electrical c ...
... VIII. Models of Electrons in Solids, and Energy Bands in solids A. Models will be used to show how electronic structure emerges from the fundamental interactions of electrons in materials, as described by quantum mechanics. B. Conduction electrons. C. The free-electron gas. D. Electrical c ...
Daniel Bernoulli
... • (v2/2 )+ P = constant (Density x velocity2 /2 )+pressure = const. As velocity of fluid increases its pressure must decrease An exchange of kinetic energy for pressure ...
... • (v2/2 )+ P = constant (Density x velocity2 /2 )+pressure = const. As velocity of fluid increases its pressure must decrease An exchange of kinetic energy for pressure ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.