GPS Content Standards
... e. Determine whether common household substances are acidic, basic, or neutral. SPS7. Students will relate transformations and flow of energy within a system. a. Identify energy transformations within a system (e.g. lighting of a match). b. Investigate molecular motion as it relates to thermal energ ...
... e. Determine whether common household substances are acidic, basic, or neutral. SPS7. Students will relate transformations and flow of energy within a system. a. Identify energy transformations within a system (e.g. lighting of a match). b. Investigate molecular motion as it relates to thermal energ ...
Med Imag. Detector Ch 3 Part
... • Consists of a scintillation phosphor and a photomultiplier coupled together through an optical contact. • Fast charged particles or gamma rays interact with the scintillation material to excite molecules which returns to their ground stae emitting optical photons.. The intensity of each scintillat ...
... • Consists of a scintillation phosphor and a photomultiplier coupled together through an optical contact. • Fast charged particles or gamma rays interact with the scintillation material to excite molecules which returns to their ground stae emitting optical photons.. The intensity of each scintillat ...
• current and current density • conductivity and resistivity • chapter 29
... charge delivered in terms of electrons current direction defined to be in which positive charges seem to move (opposite to direction of electrons - charge carriers in metals, makes no difference at macroscopic level): current in a wire from positive to negative terminal of battery ...
... charge delivered in terms of electrons current direction defined to be in which positive charges seem to move (opposite to direction of electrons - charge carriers in metals, makes no difference at macroscopic level): current in a wire from positive to negative terminal of battery ...
Quantum Numbers and Atomic Structure Honors
... A) total mass of all the protons and neutrons in an atom of Ti B) total mass of all the protons, neutrons, and electrons in an atom of Ti C) weighted average mass of the most abundant isotope of Ti D) weighted average mass of all the naturally occurring isotopes of Ti 5. The atomic mass of element A ...
... A) total mass of all the protons and neutrons in an atom of Ti B) total mass of all the protons, neutrons, and electrons in an atom of Ti C) weighted average mass of the most abundant isotope of Ti D) weighted average mass of all the naturally occurring isotopes of Ti 5. The atomic mass of element A ...
State Variables
... Transfer Variables • Transfer variables are zero unless a process occurs in which energy is transferred across the boundary of a system • Transfer variables are not associated with any given state of the system, only with changes in the state • Heat and work are transfer variables – Example of heat ...
... Transfer Variables • Transfer variables are zero unless a process occurs in which energy is transferred across the boundary of a system • Transfer variables are not associated with any given state of the system, only with changes in the state • Heat and work are transfer variables – Example of heat ...
End-semester Examination 2013 Mechanics (PHY102A/N
... Initial energy = kinetic energy = 2x½ m v02! ...
... Initial energy = kinetic energy = 2x½ m v02! ...
H - unix.eng.ua.edu
... Introduction to Quantum Chemistry LCAO Basis Set Approach Question: How do we choose the mathematical functions to construct the trial wave function? The convenient functions are called a “basis set”. If only 1 e- and 1 nucleus – exact solution of Schrödinger eq. possible (see Fig 2.3): 1s, 2s, ...
... Introduction to Quantum Chemistry LCAO Basis Set Approach Question: How do we choose the mathematical functions to construct the trial wave function? The convenient functions are called a “basis set”. If only 1 e- and 1 nucleus – exact solution of Schrödinger eq. possible (see Fig 2.3): 1s, 2s, ...
TOPIC 4 The Energy Connection
... • The four most common forms of energy are: • Chemical - potential or stored energy stored in chemicals, released when the chemicals react. • Electrical - energy of charged particles, transferred when they travel from place to place. • Mechanical - energy possessed by an object because of its motion ...
... • The four most common forms of energy are: • Chemical - potential or stored energy stored in chemicals, released when the chemicals react. • Electrical - energy of charged particles, transferred when they travel from place to place. • Mechanical - energy possessed by an object because of its motion ...
Physics_files/Unit 6 Review Part 3
... b) If dropped off the top of the pole, how much potential energy does it have 3m from the ground? c) How much kinetic energy does it have at the same height? d) How much kinetic energy does it have right before it hits the floor? 8. A cannon ball is shot from the cannon at a velocity of 40m/s straig ...
... b) If dropped off the top of the pole, how much potential energy does it have 3m from the ground? c) How much kinetic energy does it have at the same height? d) How much kinetic energy does it have right before it hits the floor? 8. A cannon ball is shot from the cannon at a velocity of 40m/s straig ...
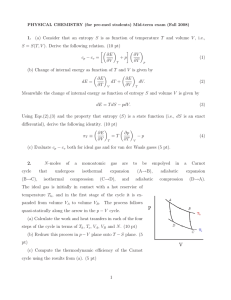
1. (a) Consider that an entropy S is as function of temperature T and
... Ei (T ) = Ci T . Note that the boxes are neither permeable nor deformable. Now we put the boxes into thermal contact with each other but still isolated from the rest of the world. We know they’ll eventually come to the same temperature when the system reaches ...
... Ei (T ) = Ci T . Note that the boxes are neither permeable nor deformable. Now we put the boxes into thermal contact with each other but still isolated from the rest of the world. We know they’ll eventually come to the same temperature when the system reaches ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.