The Binary Star Experiment What is a Binary Star? Outline
... of tension or compression of the spring.) The – sign indicates that the force is directed opposite to the tension or compression of the spring. • The ENERGY stored in a spring is equal to ½ the force times the distance, or V(r) = 1/2 k (r-ro)2 – Note that the sign now does not matter since the dista ...
... of tension or compression of the spring.) The – sign indicates that the force is directed opposite to the tension or compression of the spring. • The ENERGY stored in a spring is equal to ½ the force times the distance, or V(r) = 1/2 k (r-ro)2 – Note that the sign now does not matter since the dista ...
Thermo 2 - WordPress.com
... • To do analysis of system the system needs to be at equilibrium (e.g. no system variables are changing) • Analysis can be done at Quasi-equilibrium, which is to say the system is changing in increments at which analysis can be done. If the time change at which the analysis is done is small enough, ...
... • To do analysis of system the system needs to be at equilibrium (e.g. no system variables are changing) • Analysis can be done at Quasi-equilibrium, which is to say the system is changing in increments at which analysis can be done. If the time change at which the analysis is done is small enough, ...
Thermochemistry - Valdosta State University
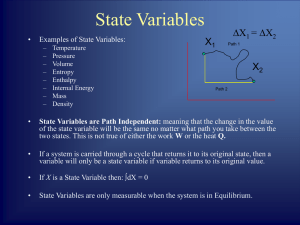
... _________________ – a process that is determined by its initial and final conditions. • “A process that is ______________________.” • ______ (w) and _____ (q) _______ state functions. • Energy change (____) ______ a state function. ...
... _________________ – a process that is determined by its initial and final conditions. • “A process that is ______________________.” • ______ (w) and _____ (q) _______ state functions. • Energy change (____) ______ a state function. ...
Document
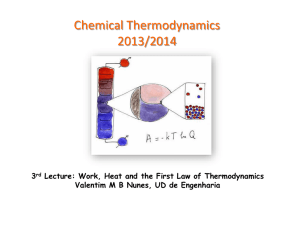
... E is the change in internal energy of a system q is the heat exchange between the system and the surroundings w is the work done on (or by) the system w = -PV when a gas expands against a constant external pressure ...
... E is the change in internal energy of a system q is the heat exchange between the system and the surroundings w is the work done on (or by) the system w = -PV when a gas expands against a constant external pressure ...
Solid State 2- Homework 7 Use the Maxwell equation
... field B inside a material is constant. But when we set the external magnetic field constant, we need to minimize a different energy: X(H,T,N) . Write an expression for X and identify it with a thermodynamic energy you know. b) Now calculate the difference between the energy X in the normal and super ...
... field B inside a material is constant. But when we set the external magnetic field constant, we need to minimize a different energy: X(H,T,N) . Write an expression for X and identify it with a thermodynamic energy you know. b) Now calculate the difference between the energy X in the normal and super ...
Ch 14.3 PPT - Using Heat
... • The disorder of a system tends to increase. – Over time, in any given system left to itself, the entropy of that system will tend to increase. • entropy: a measure of the randomness or disorder of a system • Usable energy decreases in all energy transfers. – As entropy increases – usable energy de ...
... • The disorder of a system tends to increase. – Over time, in any given system left to itself, the entropy of that system will tend to increase. • entropy: a measure of the randomness or disorder of a system • Usable energy decreases in all energy transfers. – As entropy increases – usable energy de ...
Trends in the Periodic Table
... • A: Electrons have lots of their own energy. E=hf due to their position around the nucleus. Electrons are constantly moving, very fast. • This kinetic energy overcomes the positive attraction of the nucleus. ...
... • A: Electrons have lots of their own energy. E=hf due to their position around the nucleus. Electrons are constantly moving, very fast. • This kinetic energy overcomes the positive attraction of the nucleus. ...
Chapter 3
... plane of frictionless ice. Puck A has twice the mass of puck B. Imagine that we apply the same constant force to each puck for the same interval of time dt. How do the pucks’ kinetic energies compare at the end of this interval? A. KA = 4 KB B. KA = 2 KB C. KA = KB D. KB = 2 KA E.. KB = 4 KA F. Othe ...
... plane of frictionless ice. Puck A has twice the mass of puck B. Imagine that we apply the same constant force to each puck for the same interval of time dt. How do the pucks’ kinetic energies compare at the end of this interval? A. KA = 4 KB B. KA = 2 KB C. KA = KB D. KB = 2 KA E.. KB = 4 KA F. Othe ...
File
... Periodic Trends (Chapter 5) Atomic Radius – distance from nucleus to outer electrons (PreIB only) Shielding – inner electrons “blocking” or “shielding” the valence electrons from the pull of the nucleus. Ionization Energy – energy needed to remove an electron Electronegativity – ability of an ...
... Periodic Trends (Chapter 5) Atomic Radius – distance from nucleus to outer electrons (PreIB only) Shielding – inner electrons “blocking” or “shielding” the valence electrons from the pull of the nucleus. Ionization Energy – energy needed to remove an electron Electronegativity – ability of an ...
BEZOUT IDENTITIES WITH INEQUALITY CONSTRAINTS
... Fundamentals of Physics by D. Halliday, R. Resnick and J. Walker, p. 117 : "In 1896 in Waco Texas William Crush of the 'Katy' railway parked two locomotives at opposite ends of a 6.4 km long track, fired them up, tied their throttles open, and allowed them to crash head on in front of 30,000 spectat ...
... Fundamentals of Physics by D. Halliday, R. Resnick and J. Walker, p. 117 : "In 1896 in Waco Texas William Crush of the 'Katy' railway parked two locomotives at opposite ends of a 6.4 km long track, fired them up, tied their throttles open, and allowed them to crash head on in front of 30,000 spectat ...
Lecture_2 - Department of Mathematics
... Fundamentals of Physics by D. Halliday, R. Resnick and J. Walker, p. 117 : "In 1896 in Waco Texas William Crush of the 'Katy' railway parked two locomotives at opposite ends of a 6.4 km long track, fired them up, tied their throttles open, and allowed them to crash head on in front of 30,000 spectat ...
... Fundamentals of Physics by D. Halliday, R. Resnick and J. Walker, p. 117 : "In 1896 in Waco Texas William Crush of the 'Katy' railway parked two locomotives at opposite ends of a 6.4 km long track, fired them up, tied their throttles open, and allowed them to crash head on in front of 30,000 spectat ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.