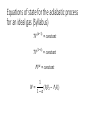* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Thermo 2 - WordPress.com
Survey
Document related concepts
Transition state theory wikipedia , lookup
Thermophotovoltaic wikipedia , lookup
Degenerate matter wikipedia , lookup
Thermal radiation wikipedia , lookup
Eigenstate thermalization hypothesis wikipedia , lookup
Heat transfer wikipedia , lookup
Temperature wikipedia , lookup
Thermal conduction wikipedia , lookup
Heat transfer physics wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Thermodynamics wikipedia , lookup
Transcript
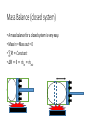
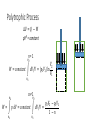
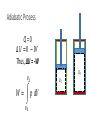
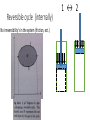
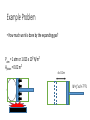
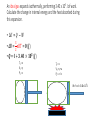
Thermodynamics 2 PH: 104: Heat and Thermodynamic Course Outline (Continued) • Thermometry • • • • • • • • • • Concept of temperature Boyle’s Law Charles’ Law Thermometers Thermocouple Calorimetry Platinum resistance scale Absolute zero Lower fixed point Temperature gradient • Thermodynamic laws and systems • • • • • • • • • • System state properties Equations of state Thermodynamic processes Work First Law of Thermodynamics Open and closed systems Internal energy Standard Temperature and Pressure Conductive heat transfer Conductivity and resistance Objectives • Understand equilibrium and quasi-equilibrium • Define the laws of thermodynamics • Define internal energy • Use definitions of work for various closed system cases Equilibrium and Equilibrium Assumptions • To do analysis of system the system needs to be at equilibrium (e.g. no system variables are changing) • Analysis can be done at Quasi-equilibrium, which is to say the system is changing in increments at which analysis can be done. If the time change at which the analysis is done is small enough, the system can be assumed to be in Quasi-equilibrium. • ALSO • For open systems (e.g. control volumes) the rate of change (mass flow) is assumed to be constant over a very short period of time. The Four Laws of Thermodynamics 0th Law: if two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other. 1st Law: is the law of conservation of energy. Energy can to be created or destroyed. There is function called internal energy that relates work and heat transfer. 2nd Law: Entropy is a function of the state of the system and cannot be reduced. Entropy is a measure of disorder or the possibility of a process occurring in nature. 3rd Law: It is impossible by any procedure, no matter how idealized, to reduce any system to the absolute zero temperature in a finite number of operations. General Idealisms of the Laws 0th Law: temperatures are relatable 1st Law: deals with quantity of energy 2nd Law: deals with quality of energy and irreversibility 3rd Law: everything ceases to exist at absolute zero Zeroth Law of Thermodynamics • If two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other. A B C • That is to say, if Ta = Tc and Tb = Tc then Ta = Tb • Are the internal energies of CO2 and H20 equal if they have the same temperature? Mechanical energy and heat equivalency • Joules Experiment (adiabatic) • Method • Dropped a weight to mix water • Many other similar experiments • Result • Slight temperature rise • Conclusions • 4.186 J = 1 cal • 4186 J = 1 kcal • 4186 J = 1 Cal (food) Mechanical energy and heat equivalency • Joules Experiment (adiabatic) • Further conclusion • Internal Energy is a measurable quantity and is relatable to work 𝑑𝑢 ∝ 𝑑𝑤 Internal energy (U) • 𝑈 is vibration and rotation energy in fluids • Sometimes loosely called heat, but this introduces a confusion between it and the energy transferred by heating ∆Q. Heating is simply the process by which energy is transferred from hot to cold. • For ideal gas and closed systems 3 2 • 𝑈 = 𝑛𝑅𝑇 • For real gases and closed system • 𝑈 = 𝑓(𝑣, 𝑇, 𝑝) • For open systems • 𝑈 = 𝐻 − 𝑃𝑉 • Where, H is entropy <- we won’t define this The First Law of Thermodynamics (Conservation of Energy) • ∆𝑈 = 𝑄 − 𝑊 • Where • Q = net heat added • W = work done by the system • ∆𝑈 = change in internal energy • Internal Energy • Is a property of the system, describes vibrational/rotational energy in a fluid • Work • Is not a property, describes mechanical energy entering (-) or leaving the system (+) • Heat • Is not a property, describes thermal energy entering (+) or leaving the system (-) Mass Balance (closed system) • A mass balance for a closed system is very easy. • Mass in = Mass out = 0 • 𝑀 = 𝐶𝑜𝑛𝑠𝑡𝑎𝑛𝑡 • ∆𝑀 = 0 = 𝑚𝑖𝑛 = 𝑚𝑜𝑢𝑡 Energy Balance (closed system) Time dependent Energy Balance: Closed System Work relationships for a closed system • Adiabatic • Isothermal • Isovolumetric • Isobaric • Polytrophic process Polytrophic Process ∆𝑈 = 𝑄 − 𝑊 pVn = constant n = 1 𝑣 2 𝑊 = 𝑐𝑜𝑛𝑠𝑡𝑎𝑛𝑡 𝑣1 𝑣2 𝑊= 𝑉2 𝑑𝑉/𝑉 = (𝑝1 𝑉1 ) ln 𝑉1 n ≠ 1𝑣 2 𝑝 𝑑𝑉 = 𝑐𝑜𝑛𝑠𝑡𝑎𝑛𝑡 𝑣1 𝑣1 𝑝2 𝑉2 − 𝑝1 𝑉1 𝑑𝑉/𝑉 = 1 −𝑛 Adiabatic Process Q=0 ∆𝑈 = 0 − 𝑊 Thus, ΔU = -W U2 𝑣2 𝑊= U1 𝑝 𝑑𝑉 𝑣1 Equations of state for the adiabatic process for an ideal gas (Syllabus) 𝑇𝑉 (𝛼−1) = 𝑐𝑜𝑛𝑠𝑡𝑎𝑛𝑡 𝑇𝑃(1−∝) = 𝑐𝑜𝑛𝑠𝑡𝑎𝑛𝑡 𝑃𝑉 𝛼 = 𝑐𝑜𝑛𝑠𝑡𝑎𝑛𝑡 1 𝑊= (𝑃2 𝑉2 − 𝑃1 𝑉1 ) 1−𝛼 P1 Isobaric Process W=fxd W = PAd Thus, W = PΔV OR 𝑣2 𝑊= 𝑣2 𝑝 𝑑𝑉 = 𝑝 𝑣1 𝑑𝑉 = 𝑝(𝑉2 − 𝑉1 ) 𝑣1 = P2 V1 Isovolumetric Process Volume is constant W = PΔV Thus, W = 0 = V2 Isothermal Process (for ideal gasses in closed systems) ∆𝑈 = 𝑄 − 𝑊 ∆𝑈 = 0 Thus, 𝑄 = 𝑊 𝑃𝑉 = 𝑛𝑅𝑇 𝑛 = 𝐶𝑜𝑛𝑠𝑡𝑎𝑛𝑡 𝑅 = 𝐶𝑜𝑛𝑠𝑡𝑎𝑛𝑡 𝑇 = 𝐶𝑜𝑛𝑠𝑡𝑎𝑛𝑡 Thus, 𝑃𝑉 = 𝐶𝑜𝑛𝑠𝑡𝑎𝑛𝑡 T1 = T2 Isothermal Process 𝑂𝑅 n=1 n pV = constant p𝑉 = 𝐶𝑜𝑛𝑠𝑡𝑎𝑛𝑡 𝑣2 𝑊= 𝑣2 𝑝 𝑑𝑉 = 𝑐𝑜𝑛𝑠𝑡𝑎𝑛𝑡 𝑣1 𝑣1 𝑉2 𝑑𝑉/𝑉 = (𝑝1 𝑉1 ) ln 𝑉1 T1 = T2 Cycles • Are just a set of processes in series 1-2-3-4-1-2-3-4-1-2-3-4, ect. • Two major types: Power Cycle and refrigeration and heat pump cycles • Engines operate on Power Cycle • Carnot cycle • Otto cycle Examples of why aren’t processes or cycles 100% efficient (irreversibility) • Heat transfer through a finite temperature difference • Unrestrained expansion of a gas or liquid to a lower pressure • Spontaneous chemical reaction • Spontaneous mixing of matter at different compositions or states • Friction- sliding friction as well as friction in the flow of fluids • Electric current flow through a resistance • Magnetization of polarization with hysteresis • Inelastic deformation Reversible cycle (internally) No irreversibility's in the system (friction, ect.) 1 ↔ 2 Self study syllabus topic • The relationship between the principal specific heat capacities 𝐶𝑣 𝑎𝑛𝑑 𝐶𝑝 • Conductive heat transfer • Thermal conductivity and resistance Second law not on syllabus • But, it is the law that proves no REAL process or cycle is 100% efficient Second Law of Thermodynamics • Heat can flow spontaneously from a hot object to a cold object; heat will not flow spontaneously from a cold object to a hot object. • Some processes adhere to the First Law of Thermodynamics but do not happen in both directions (i.e. a cup hitting the ground and breaking or mixing salt and pepper) • Things tend to spontaneously go from a state of order to disorder (increasing entropy) but not the other direction. Example Problem • How much work is done by the expanding gas? Patm = 1 atm or 1.013 x 10⁵ N/m² Apiston = 0.02 m² d = 0.1 m W = f x d = ??? J Example problem • An ideal gas expands isothermally, performing 3.40 x 103 J of work. Calculate the change in internal energy and the heat absorbed during this expansion. An ideal gas expands isothermally, performing 3.40 x 103 J of work. Calculate the change in internal energy and the heat absorbed during this expansion. • ∆𝑈 = 𝑄 − 𝑊 • ∆𝑈 = 𝑄 − 3.40 × 103 (𝐽) • Do I remember the qualifications of isothermal expansion? T1 = x V1 = y P1 = z T2 = x V2 = y + a P2 = ??? W = f x d = 3.40 x 103 J An ideal gas expands isothermally, performing 3.40 x 103 J of work. Calculate the change in internal energy and the heat absorbed during this expansion. • ∆𝑈 = 𝑄 − 𝑊 • 𝑄 = ∆𝑈 + 3.40 × 103 (𝐽) • How do gases expand? How do gases expand without changing temp? T1 = x V1 = y P1 = z T2 = x V2 = y + a P2 = ??? W = f x d = 3.40 x 103 J An ideal gas expands isothermally, performing 3.40 x 103 J of work. Calculate the change in internal energy and the heat absorbed during this expansion. • ∆𝑈 = 𝑄 − 𝑊 • ∆𝑼 = 3 𝑛𝑅𝑇 2 = 𝟎 (𝑱) • 𝑸 = 0 + 𝟑. 𝟒𝟎 × 𝟏𝟎𝟑 (𝑱) T1 = x V1 = y P1 = z T2 = x V2 = y + a P2 = z - b W = f x d = 3.40 x 103 J We will use a Mass Balance and Energy Balance (first law) and apply the equations to solve for tank pressure at time (t2) if pump power, flow rate, Pt1 and surrounding variables were known. 𝑃𝑡2 = ? Polytrophic example 1. Four kilograms of a gas is contained within a piston-cylinder assembly. The gas undergoes a process for which the pressurevolume relationship is 𝑝𝑉 1.5 = 𝑐𝑜𝑛𝑠𝑡𝑎𝑛𝑡. The initial pressure is 3 bar, the initial volume is 0.1 𝑚3 . And the final volume is 0.2 𝑚3 . The change in specific internal energy of the gas in the process is 𝑢2 − 𝑘𝐽 𝑢1 = −4.6 . There are no significant changes in the kinetic or 𝑘𝑔 potential energy. Determine the net heat transfer for the process, in kJ. Second Law of Thermodynamics • Heat can flow spontaneously from a hot object to a cold object; heat will not flow spontaneously from a cold object to a hot object. • Some processes adhere to the First Law of Thermodynamics but do not happen in both directions (i.e. a cup hitting the ground and breaking or mixing salt and pepper) • Things tend to spontaneously go from a state of order to disorder (increasing entropy) but not the other direction. Reversible thermodynamic process Thermodynamic intensive and extensive properties, variables • Intensive do not change when identical systems are added • Extensive depend on the amount of the substance in the system Intensive Extensive Temperature, T Volume, V Pressure, P Mass, m Density, ρ Area, A Composition Group Class Homework 1. Which is larger 1 °F or 1 C°? 2. The units for the coefficient of linear expansion α are (°C)-1 and there is no mention of a length unit such as meters. Would the expansion coefficient change if we used feet or millimeters instead of meters? 3. Long steam pipes that are fixed at the ends often have a section in the shape of a U. Why? Superheating • When a gas is heated to above the critical temperature the vapor, gas or fluid is said to be super heated. This a metastable phase that usually has a transient existence (e.g. short life). Supersaturation • When a gas has more vapor than the SVP do to high pressures. This processes is also unstable and often short lived. • What does supersaturation look like in our environment? • What is the dew point? Group homework 1. 15 below zero on the Celsius scale is what temperature Fahrenheit and kelvin? 2. 15 below zero on the Fahrenheit scale is what temperature in Celsius and kelvin? 3. The Eiffel Tower is 300 m tall and made of wrought iron. Estimate how much the height changes from July (ave. 25 °C) to January (ave. 2 °C). 4. The density of water at 4 °C is 1000 kg/m³. What is water’s density at 94 °C? 5. Absolute zero is what temperature on the Fahrenheit scale? 6. At (a) atmospheric pressure, in what phases can CO2 exist? (b) For what range of pressure and temperatures can CO2 be a liquid? 7. Extra credit 1. What is the approximate temperature inside a pressure cooker if the water is boiling at a temperature of 120 ° C? Assume no air escaped during the heating process, which started at 20 ° C.