Midterm review
... Boundary conditions: Ends tied down standing wave More nodes higher energy 4. 2D waves for a square or round drum have degenerate sets (i.e. two or more waves have the same energy. They are the same wave only rotate in space. Schrödinger Equations: 3D wavefunctions (r) or Orbitals ...
... Boundary conditions: Ends tied down standing wave More nodes higher energy 4. 2D waves for a square or round drum have degenerate sets (i.e. two or more waves have the same energy. They are the same wave only rotate in space. Schrödinger Equations: 3D wavefunctions (r) or Orbitals ...
lecture
... • Electric work and energy Energy of a charge distribution Energy density in terms of E field • Field lines and equipotentials Drawing field lines Flux x flow analogy • Poisson’s equation Curvature of function Green’s functions ...
... • Electric work and energy Energy of a charge distribution Energy density in terms of E field • Field lines and equipotentials Drawing field lines Flux x flow analogy • Poisson’s equation Curvature of function Green’s functions ...
topic-2.doc
... Electrons are in orbit around the nucleus, are involved in chemical reactions. o Orbital: three-dimensional space where an electron will most likely be found 90% of the time o First energy level: one s orbital, holds 2 electrons o Second energy level: one s and three p orbitals, holds 8 electrons Ch ...
... Electrons are in orbit around the nucleus, are involved in chemical reactions. o Orbital: three-dimensional space where an electron will most likely be found 90% of the time o First energy level: one s orbital, holds 2 electrons o Second energy level: one s and three p orbitals, holds 8 electrons Ch ...
Relationships Between Heat and Work
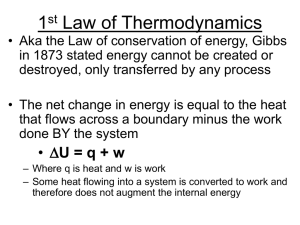
... • Internal energy is constant in a constanttemperature process • Isothermal process – a thermodynamic process that takes place at constant temperature and in which the internal energy of a system remains unchanged – Similar to a balloon expanding as the pressure drops before a storm hits • The ballo ...
... • Internal energy is constant in a constanttemperature process • Isothermal process – a thermodynamic process that takes place at constant temperature and in which the internal energy of a system remains unchanged – Similar to a balloon expanding as the pressure drops before a storm hits • The ballo ...
Chapter 4, 5 and 6 Review: Atomic Theory, the Electron and the
... chalcogens, halogens, noble gases, inner transition metals, transition metal, group or family, period, periodic law, octet rule, atomic radius, ionic radius, ionization energy, electronegativity,, valence electrons. ...
... chalcogens, halogens, noble gases, inner transition metals, transition metal, group or family, period, periodic law, octet rule, atomic radius, ionic radius, ionization energy, electronegativity,, valence electrons. ...
New Microsoft Office Word Document
... Universe:- System along with all the surroundings Boundary:- Walls that separate System from Surroundings Equilibrium:- A state of dynamics wherein all observable properties are constant Thermodynamic Equilibrium:- A system in which all macroscopic properties do not undergo any change with time Ther ...
... Universe:- System along with all the surroundings Boundary:- Walls that separate System from Surroundings Equilibrium:- A state of dynamics wherein all observable properties are constant Thermodynamic Equilibrium:- A system in which all macroscopic properties do not undergo any change with time Ther ...
Matter and Energy unit review answer key
... molecules, their movement, and their shape, and volume. ...
... molecules, their movement, and their shape, and volume. ...
Lecture 5 - Thermodynamics II
... For internal energy of a thing: dU = dqtot – PdV; determining this at constant volume dU = CVdT where CV is the heat required to raise T by 1°C ...
... For internal energy of a thing: dU = dqtot – PdV; determining this at constant volume dU = CVdT where CV is the heat required to raise T by 1°C ...
Thermochemistry www.AssignmentPoint.com Thermochemistry is
... of energy in the form of chemical bonds. The subject commonly includes calculations of such quantities as heat capacity, heat of combustion, heat of formation, enthalpy, entropy, free energy, and calories. ...
... of energy in the form of chemical bonds. The subject commonly includes calculations of such quantities as heat capacity, heat of combustion, heat of formation, enthalpy, entropy, free energy, and calories. ...
Lecture 5
... the figure below, Q = 50 cal and W = 20 cal. Along path ibf, Q = 36 cal. (a) What is W along path ibf? (b) If W = -13 cal for the return path fi, what is Q for this path? (c) If Eint,i = 10 cal, what is Eint,f? If Eint,b = 22 cal, what is Q for (d) path ib and (e) path bf? ...
... the figure below, Q = 50 cal and W = 20 cal. Along path ibf, Q = 36 cal. (a) What is W along path ibf? (b) If W = -13 cal for the return path fi, what is Q for this path? (c) If Eint,i = 10 cal, what is Eint,f? If Eint,b = 22 cal, what is Q for (d) path ib and (e) path bf? ...
241 Lecture 11
... • Two systems are said to be in thermal equilibrium if there is no net flow of heat between them when they are brought into thermal contact. • Temperature is the indicator of thermal equilibrium • Two systems individually in thermal equilibrium with a third system are in thermal equilibrium with ...
... • Two systems are said to be in thermal equilibrium if there is no net flow of heat between them when they are brought into thermal contact. • Temperature is the indicator of thermal equilibrium • Two systems individually in thermal equilibrium with a third system are in thermal equilibrium with ...
Thermodynamic principles. - med.muni
... • Cyclic process: the initial and final states of the system are identical (but not necessarily the surroundings) • Sign convention: energy given to a system and work done by an external force on the system are considered to be positive, energy lost from the system to its surroundings and work done ...
... • Cyclic process: the initial and final states of the system are identical (but not necessarily the surroundings) • Sign convention: energy given to a system and work done by an external force on the system are considered to be positive, energy lost from the system to its surroundings and work done ...
Document
... Max Planck (1900) solved the paradox of the blackbody radiation. Classical Physics assumed that atoms and molecules could emit (or absorb) any arbitrary amount of radiant energy. He proposed that this energy could be emitted or absorbed only in discrete quantities. He gave the name of quantum to th ...
... Max Planck (1900) solved the paradox of the blackbody radiation. Classical Physics assumed that atoms and molecules could emit (or absorb) any arbitrary amount of radiant energy. He proposed that this energy could be emitted or absorbed only in discrete quantities. He gave the name of quantum to th ...
FRCRIII - hullrad Radiation Physics
... n: number of photons removed from the beam N: number of photons incident on the material Δx: thickness of the material (cm) ...
... n: number of photons removed from the beam N: number of photons incident on the material Δx: thickness of the material (cm) ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.