experiment 18: flame tests for metals
... Fireworks capture our attention with their beautiful colors and controlled explosions, both of which link directly to fundamental concepts taught in basic chemistry classes. The media resources featured in this lesson provide a visually rich way to tie together spectral chemistry, combustion, and th ...
... Fireworks capture our attention with their beautiful colors and controlled explosions, both of which link directly to fundamental concepts taught in basic chemistry classes. The media resources featured in this lesson provide a visually rich way to tie together spectral chemistry, combustion, and th ...
ppt notes
... different than A and BA element Atoms of element A and B can be can be physically chemically combined mixed together as a compound ...
... different than A and BA element Atoms of element A and B can be can be physically chemically combined mixed together as a compound ...
DEFINING THE ATOM - BradyMathScience
... ________ 19. How do the isotopes hydrogen-2 and hydrogen-3 differ? a. Hydrogen-3 has one more electron than hydrogen-2. b. Hydrogen-3 has two neutrons. c. Hydrogen-2 has three protons. d. Hydrogen-2 has no protons. ________ 20. The number 80 in the name bromine-80 represents a. the atomic number. b. ...
... ________ 19. How do the isotopes hydrogen-2 and hydrogen-3 differ? a. Hydrogen-3 has one more electron than hydrogen-2. b. Hydrogen-3 has two neutrons. c. Hydrogen-2 has three protons. d. Hydrogen-2 has no protons. ________ 20. The number 80 in the name bromine-80 represents a. the atomic number. b. ...
5 Early Atomic Theory and Structure Chapter Outline Early Theories
... 3. Atoms of different elements differ in their mass and size. 4. Compounds are formed by combining two or more atoms of different elements. 5. Atoms combine to form compounds in simple whole number ratios. ...
... 3. Atoms of different elements differ in their mass and size. 4. Compounds are formed by combining two or more atoms of different elements. 5. Atoms combine to form compounds in simple whole number ratios. ...
4 Structure of The Atom
... 1. Experiments on static electricity have proved that seemingly electrically neutral matter consists of electrically charged particles, such that positive charges in it are equal to the negative charges. 2. The electron was discovered by J.J. Thomson. 3. The proton was discovered by E. Gold ...
... 1. Experiments on static electricity have proved that seemingly electrically neutral matter consists of electrically charged particles, such that positive charges in it are equal to the negative charges. 2. The electron was discovered by J.J. Thomson. 3. The proton was discovered by E. Gold ...
AP Chemistry
... that you have 12 pencils or 25 bottles of soda or 150 marbles. When you measure something, however, you obtain a number that is not exact. For example, you can determine that a beaker has a mass of 250 g by weighing it on a scale. Using a different scale might give you a mass of 249.9 g for the same ...
... that you have 12 pencils or 25 bottles of soda or 150 marbles. When you measure something, however, you obtain a number that is not exact. For example, you can determine that a beaker has a mass of 250 g by weighing it on a scale. Using a different scale might give you a mass of 249.9 g for the same ...
chem1a_ch02_lecture - Santa Rosa Junior College
... compounds dissolve in water. For example, when gaseous hydrogen chloride (HCl) dissolves in water, it forms a solution called hydrochloric acid. Prefix hydro- + anion nonmetal root + suffix -ic + the word acid hydro + chlor + ic + acid ...
... compounds dissolve in water. For example, when gaseous hydrogen chloride (HCl) dissolves in water, it forms a solution called hydrochloric acid. Prefix hydro- + anion nonmetal root + suffix -ic + the word acid hydro + chlor + ic + acid ...
chem1a_ch02_lecture - Santa Rosa Junior College
... compounds dissolve in water. For example, when gaseous hydrogen chloride (HCl) dissolves in water, it forms a solution called hydrochloric acid. Prefix hydro- + anion nonmetal root + suffix -ic + the word acid hydro + chlor + ic + acid ...
... compounds dissolve in water. For example, when gaseous hydrogen chloride (HCl) dissolves in water, it forms a solution called hydrochloric acid. Prefix hydro- + anion nonmetal root + suffix -ic + the word acid hydro + chlor + ic + acid ...
atomic mass - Belle Vernon Area School District
... • Thomson believed that these particles were therefore the ultimate building blocks of matter “We have in the cathode rays matter in a new state, a state in which the subdivision of matter is carried very much further . . . a state in which all matter . . . is of one and the same kind; this matter ...
... • Thomson believed that these particles were therefore the ultimate building blocks of matter “We have in the cathode rays matter in a new state, a state in which the subdivision of matter is carried very much further . . . a state in which all matter . . . is of one and the same kind; this matter ...
Semester 1 exam review
... 18. The human bladder can hold up to 2 cups of urine. (for those of you that need to go to the bathroom all the time) How many liters is this? Review questions for test on Ch. 3 1. What is the kinetic theory of matter? 2. What are the two definitions of a gas? 3. What makes air pressure on the atomi ...
... 18. The human bladder can hold up to 2 cups of urine. (for those of you that need to go to the bathroom all the time) How many liters is this? Review questions for test on Ch. 3 1. What is the kinetic theory of matter? 2. What are the two definitions of a gas? 3. What makes air pressure on the atomi ...
Topic 9 - Anderson High School
... A species is oxidized when it loses electrons. – Here, zinc loses two electrons to go from neutral zinc metal to the Zn2+ ion. ...
... A species is oxidized when it loses electrons. – Here, zinc loses two electrons to go from neutral zinc metal to the Zn2+ ion. ...
Atomic Timeline There are small, negatively charged particles inside
... Atomic Timeline The table below contains a number of statements connected to major discoveries in the development of the atomic theory. ...
... Atomic Timeline The table below contains a number of statements connected to major discoveries in the development of the atomic theory. ...
Elements and the Periodic Table
... around the nucleus of a hydrogen atom. Schrödinger, on the other hand, used the energy of electrons to calculate the shape of the electron clouds around the nucleus. While the electron cloud model of the atom does not show any easily visible energy levels, Schrödinger’s equation did allow him to fin ...
... around the nucleus of a hydrogen atom. Schrödinger, on the other hand, used the energy of electrons to calculate the shape of the electron clouds around the nucleus. While the electron cloud model of the atom does not show any easily visible energy levels, Schrödinger’s equation did allow him to fin ...
Dalton`s Atomic Theory
... the atom. This later became known as Dalton’s atomic theory. The general tenets of this theory were as follows: • All matter is composed of extremely small particles called atoms. • Atoms of a given element are identical in size, mass, and other properties. Atoms of different elements differ in size ...
... the atom. This later became known as Dalton’s atomic theory. The general tenets of this theory were as follows: • All matter is composed of extremely small particles called atoms. • Atoms of a given element are identical in size, mass, and other properties. Atoms of different elements differ in size ...
FINAL REVIEW - Normal Community High School Chemistry
... surrounding freely mobile electrons. Most metals contribute more than one mobile electron per atom. Bailar, Jr, Moeller, Kleinberg, Guss, Castellion, Metz, Chemistry, 1984, page 245 ...
... surrounding freely mobile electrons. Most metals contribute more than one mobile electron per atom. Bailar, Jr, Moeller, Kleinberg, Guss, Castellion, Metz, Chemistry, 1984, page 245 ...
Topic 2.3 The Atom Electron Configuration
... down as much information about each one as you can remember. What can you say about the numbers of protons and electrons in an atom? ...
... down as much information about each one as you can remember. What can you say about the numbers of protons and electrons in an atom? ...
atom
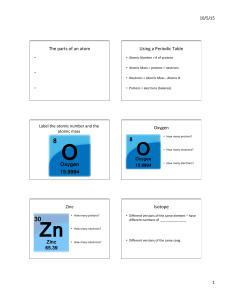
... The atomic masses of its three stable isotopes, 8O (99.757 percent), 8O (0.038 percent),188O (0.205 percent), are 15.9949, 16.9991, and 17.9992 amu, respectively. Calculate the average atomic mass of oxygen using the relative abundances given in parentheses. Strategy Each isotope contributes to the ...
... The atomic masses of its three stable isotopes, 8O (99.757 percent), 8O (0.038 percent),188O (0.205 percent), are 15.9949, 16.9991, and 17.9992 amu, respectively. Calculate the average atomic mass of oxygen using the relative abundances given in parentheses. Strategy Each isotope contributes to the ...
Final "I Can Statements" Answer Key
... If you can do all the things listed below, you are ready for the Unit 2 test. Place a checkmark next to each item that you can do! If a sample problem is given, complete it as evidence. _____1. I can still do everything from Unit 1. Definitions: atom – smallest particle of matter that retains the pr ...
... If you can do all the things listed below, you are ready for the Unit 2 test. Place a checkmark next to each item that you can do! If a sample problem is given, complete it as evidence. _____1. I can still do everything from Unit 1. Definitions: atom – smallest particle of matter that retains the pr ...