Chapter 15 The Tricarboxylic Acid Cycle
... Regulation of Amino Acids catabolism in TCA Cycle Entering TCA cycle of amino acids via -ketoglutarate and succinyl-CoA has no apparent regulation site during their conversion to oxaloacetate. : Since oxaloacetate can not go further without acetyl-CoA, pyruvate dehydrogenase will be responsible for ...
... Regulation of Amino Acids catabolism in TCA Cycle Entering TCA cycle of amino acids via -ketoglutarate and succinyl-CoA has no apparent regulation site during their conversion to oxaloacetate. : Since oxaloacetate can not go further without acetyl-CoA, pyruvate dehydrogenase will be responsible for ...
The Krebs Cycle - Advanced
... Aerobic respiration begins with the entry of the product of glycolysis, pyruvate, into the mitochondria. For each initial glucose molecules, two pyruvate molecules will enter the mitochondria. Pyruvate, however, is not the molecule that enters the Krebs cycle. Prior to entry into this cycle, pyruvat ...
... Aerobic respiration begins with the entry of the product of glycolysis, pyruvate, into the mitochondria. For each initial glucose molecules, two pyruvate molecules will enter the mitochondria. Pyruvate, however, is not the molecule that enters the Krebs cycle. Prior to entry into this cycle, pyruvat ...
03-232 Exam III 2013 Name:__________________________
... Choice A: The mainchain Hbond donors and acceptors will be forming H-bonds with water in the unfolded protein. When the protein folds those H-bonds are broken at a cost of +20 kJ/mol, which is energetically unfavorable. Since there are no H-bond donors and acceptors in the membrane, the protein has ...
... Choice A: The mainchain Hbond donors and acceptors will be forming H-bonds with water in the unfolded protein. When the protein folds those H-bonds are broken at a cost of +20 kJ/mol, which is energetically unfavorable. Since there are no H-bond donors and acceptors in the membrane, the protein has ...
Student Module_4
... energy are called metabolism. • Two interrelated energy-producing systems: – Aerobic: requiring oxygen. – Anaerobic: not requiring oxygen • Inefficient; generates lactic acid that can be converted into an energy substrate. • The energy used by cells is called ATP. ATP production is synthesized prima ...
... energy are called metabolism. • Two interrelated energy-producing systems: – Aerobic: requiring oxygen. – Anaerobic: not requiring oxygen • Inefficient; generates lactic acid that can be converted into an energy substrate. • The energy used by cells is called ATP. ATP production is synthesized prima ...
Physiological effects of exercise
... about by stimulation from the noradrenergic sympathetic nervous system. The increase in heart rate is also mediated by vagal inhibition and is sustained by autonomic sympathetic responses and carbon dioxide acting on the medulla. The efficacy of systolic contraction is particularly important in trai ...
... about by stimulation from the noradrenergic sympathetic nervous system. The increase in heart rate is also mediated by vagal inhibition and is sustained by autonomic sympathetic responses and carbon dioxide acting on the medulla. The efficacy of systolic contraction is particularly important in trai ...
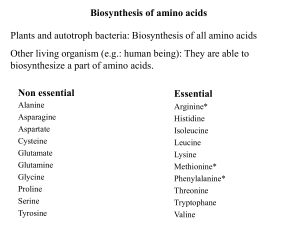
Biosynthesis of amino acids
... Nucleosides are derivatives of purines and pyrimidines that have a sugar linked to a ring nitrogen. Numerals with a prime (eg, 2′ or 3′) distinguish atoms of the sugar from those of the heterocyclic base. ...
... Nucleosides are derivatives of purines and pyrimidines that have a sugar linked to a ring nitrogen. Numerals with a prime (eg, 2′ or 3′) distinguish atoms of the sugar from those of the heterocyclic base. ...
An Overview of the Citric Acid Cycle
... Stoichiometry of the Citric Acid Cycle 1. Two carbon atoms enter the cycle in the condensation of an acetyl unit (from acetyl CoA) with oxaloacetate. Two carbon atoms leave the cycle in the form of CO2 in the successive decarboxylations catalyzed by isocitrate dehydrogenase and a-ketoglutarate deh ...
... Stoichiometry of the Citric Acid Cycle 1. Two carbon atoms enter the cycle in the condensation of an acetyl unit (from acetyl CoA) with oxaloacetate. Two carbon atoms leave the cycle in the form of CO2 in the successive decarboxylations catalyzed by isocitrate dehydrogenase and a-ketoglutarate deh ...
FREE Sample Here
... Arsenate is a toxic ion that can interfere with both glycolysis and oxidative phosphorylation. Arsenate resembles Pi (inorganic phosphate) and can replace it in many enzymatic reactions. One such reaction is catalyzed by glyceraldehyde 3-phosphate dehydrogenase in step 6 of glycolysis. Upon completi ...
... Arsenate is a toxic ion that can interfere with both glycolysis and oxidative phosphorylation. Arsenate resembles Pi (inorganic phosphate) and can replace it in many enzymatic reactions. One such reaction is catalyzed by glyceraldehyde 3-phosphate dehydrogenase in step 6 of glycolysis. Upon completi ...
Non-competitive
... Apoenzyme – the polypeptide portion of an enzyme Cofactor – non protein portion of an enzyme May be a metal ion such as Zn2+ of Mg2+ May also be an organic molecule such as vitamin B or heme – called a coenzyme Substrate – the molecule an enzyme acts on Activation – any process that initiates or in ...
... Apoenzyme – the polypeptide portion of an enzyme Cofactor – non protein portion of an enzyme May be a metal ion such as Zn2+ of Mg2+ May also be an organic molecule such as vitamin B or heme – called a coenzyme Substrate – the molecule an enzyme acts on Activation – any process that initiates or in ...
LECTURE TEST PACKET #3
... the 2 NADH molecules produced in glycolysis must travel into the mitochondria to be processed in the electron transport system. This doesn’t occur in the prokaryotic cells where all of the chemical reactions occur side by side. In eukaryotes, each NADH that moves into the mitochondria will need to u ...
... the 2 NADH molecules produced in glycolysis must travel into the mitochondria to be processed in the electron transport system. This doesn’t occur in the prokaryotic cells where all of the chemical reactions occur side by side. In eukaryotes, each NADH that moves into the mitochondria will need to u ...
Nucleotide Catabolism
... Very little of the nucleic acids ingested in our diets become incorporated into the nucleic acids in our cells. Most of the ingested bases are excreted. The salvage pathways are to continuously recycle cellular constituents not incorporated dietary bases. IV. Purine Nucleotide Catabolism The purine ...
... Very little of the nucleic acids ingested in our diets become incorporated into the nucleic acids in our cells. Most of the ingested bases are excreted. The salvage pathways are to continuously recycle cellular constituents not incorporated dietary bases. IV. Purine Nucleotide Catabolism The purine ...
Enzyme
... cell behave like reversible noncompetitive inhibitors. * Allosteric regulation --- Is the term used to describe any case in which a protein’s function at one site is affected by binding of a regulatory molecule at another site. ...
... cell behave like reversible noncompetitive inhibitors. * Allosteric regulation --- Is the term used to describe any case in which a protein’s function at one site is affected by binding of a regulatory molecule at another site. ...
Chapter 3
... Exercising skeletal muscles produce lactic acid. However, once produced in the body, lactic acid is rapidly converted to its conjugate base, lactate. Muscle cells can produce ATP by any one or a combination of three metabolic pathways: (1) ATPPC system, (2) glycolysis, (3) oxidative ATP producti ...
... Exercising skeletal muscles produce lactic acid. However, once produced in the body, lactic acid is rapidly converted to its conjugate base, lactate. Muscle cells can produce ATP by any one or a combination of three metabolic pathways: (1) ATPPC system, (2) glycolysis, (3) oxidative ATP producti ...
UNIT 11. CATABOLISM OF GLUCOSE • Aerobic glycolysis: scheme
... catalyzed by glyceraldehyde-3-phosphate dehydrogenase (oxidative phosphorylation), pyruvate kinase and phosphoglycerate kinase (substrate level phosphorylation). When glucose is oxidized completely to CO2 and H2O (Fig. 199 (B)), 36 or 38 moles of ATP are generated. In this case pyruvate may enter mi ...
... catalyzed by glyceraldehyde-3-phosphate dehydrogenase (oxidative phosphorylation), pyruvate kinase and phosphoglycerate kinase (substrate level phosphorylation). When glucose is oxidized completely to CO2 and H2O (Fig. 199 (B)), 36 or 38 moles of ATP are generated. In this case pyruvate may enter mi ...
Metabolism of RBC
... • The red blood cell is simple in terms of its structure and function, consisting principally of a concentrated solution of hemoglobin surrounded by a membrane. The erythrocyte membrane is flexible because of its cytoskeletal structure. • The red cell contains a battery of cytosolic enzymes, such as ...
... • The red blood cell is simple in terms of its structure and function, consisting principally of a concentrated solution of hemoglobin surrounded by a membrane. The erythrocyte membrane is flexible because of its cytoskeletal structure. • The red cell contains a battery of cytosolic enzymes, such as ...
08_Lecture_Presentation_PC
... helps control metabolism • Chemical chaos would result if a cell’s metabolic pathways were not tightly regulated • A cell does this by switching on or off the genes that encode specific enzymes or by regulating the activity of enzymes © 2011 Pearson Education, Inc. ...
... helps control metabolism • Chemical chaos would result if a cell’s metabolic pathways were not tightly regulated • A cell does this by switching on or off the genes that encode specific enzymes or by regulating the activity of enzymes © 2011 Pearson Education, Inc. ...
AP Biology “What You Need To KNOW” for Free Energy
... Be able to model and explain how ATP is used to complete tasks “work” in the cell. Explain a specific chemical, mechanical, and transport mechanism using ATP as an energy coupling molecule. Explain energy coupling in detail utilizing ΔG = -7.3 kcal/mol . How much work can it do??? Compare / Contrast ...
... Be able to model and explain how ATP is used to complete tasks “work” in the cell. Explain a specific chemical, mechanical, and transport mechanism using ATP as an energy coupling molecule. Explain energy coupling in detail utilizing ΔG = -7.3 kcal/mol . How much work can it do??? Compare / Contrast ...
The energy equivalents of ATP and the energy values of food
... glycerol used in the computation of ATP yields were direct, i.e. not via the storage forms glycogen, fat and protein. Oxidation of protein via gluconeogenesis was considered as an option. Several assumptions were made : ATP expenditure within the mitochondrial matrix, except for substrate activation ...
... glycerol used in the computation of ATP yields were direct, i.e. not via the storage forms glycogen, fat and protein. Oxidation of protein via gluconeogenesis was considered as an option. Several assumptions were made : ATP expenditure within the mitochondrial matrix, except for substrate activation ...
EndoMet Supplements
... Betaine HCl and Pepsin contains specific nutrients required for digestion of protein in the stomach. Hydrochloric acid is necessary to activate pepsin, the major proteolytic enzyme in the stomach. Without adequate hydrochloric acid and pepsin, protein digestion is impaired. Poor protein digestion c ...
... Betaine HCl and Pepsin contains specific nutrients required for digestion of protein in the stomach. Hydrochloric acid is necessary to activate pepsin, the major proteolytic enzyme in the stomach. Without adequate hydrochloric acid and pepsin, protein digestion is impaired. Poor protein digestion c ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.