Purine nucleotide synthesis De novo
... The regulation of de novo pyrimidine synthesis CPS II is activated by PRPP and inhibited by UTP As cells approach the S-phase of the cell cycle CPS II becomes more sensitive to activation by PRPP and less sensitive? to inhibition by UTP phosphorylation of CPS II by MAP kinase at a specific site le ...
... The regulation of de novo pyrimidine synthesis CPS II is activated by PRPP and inhibited by UTP As cells approach the S-phase of the cell cycle CPS II becomes more sensitive to activation by PRPP and less sensitive? to inhibition by UTP phosphorylation of CPS II by MAP kinase at a specific site le ...
Slides - WordPress.com
... rTCA cycle was originally discovered in green sulfur phototrophs and has since been identified in a variety of chemoautotrophs rTCA cycle specific enzymes are 2-oxoglutarate:ferredoxin oxidoreductase (Oor), fumarate reductase (Frd), and ATP citrate lyase (Acl) rTCA cycle pathway tends to be in ...
... rTCA cycle was originally discovered in green sulfur phototrophs and has since been identified in a variety of chemoautotrophs rTCA cycle specific enzymes are 2-oxoglutarate:ferredoxin oxidoreductase (Oor), fumarate reductase (Frd), and ATP citrate lyase (Acl) rTCA cycle pathway tends to be in ...
chapter 8 section 3 notes
... During the light-independent reactions, commonly referred to as the Calvin cycle, plants use the energy that ATP and NADPH contains to build stable high-energy carbohydrate compounds that can be stored for a long time. ...
... During the light-independent reactions, commonly referred to as the Calvin cycle, plants use the energy that ATP and NADPH contains to build stable high-energy carbohydrate compounds that can be stored for a long time. ...
Chem*3560 Lecture 23: Phospholipid Biosynthesis
... are described as glycerolipids . The synthesis pathway starts by reducing dihydroxyacetone phosphate to glycerol phosphate, with NADH as the reductant (Lehninger p789). NAD+ dependent glycerol phosphate dehydrogenase dihydroxyacetone phosphate + NADH + H+ → L-glycerol-3-phosphate + NAD + (Compare th ...
... are described as glycerolipids . The synthesis pathway starts by reducing dihydroxyacetone phosphate to glycerol phosphate, with NADH as the reductant (Lehninger p789). NAD+ dependent glycerol phosphate dehydrogenase dihydroxyacetone phosphate + NADH + H+ → L-glycerol-3-phosphate + NAD + (Compare th ...
The Enzymic Activity of the Outer Shell of
... aerobes and which, in contrast to the preparations from lactobacilli, contained enzymes associated with oxygen utilization mediated by the cytochrome hydrogen transport system. The bound ATPase had an optimum at pH 6.0 and was not markedly inhibited by ouabain or oligomycin: it was stimulated by 2,& ...
... aerobes and which, in contrast to the preparations from lactobacilli, contained enzymes associated with oxygen utilization mediated by the cytochrome hydrogen transport system. The bound ATPase had an optimum at pH 6.0 and was not markedly inhibited by ouabain or oligomycin: it was stimulated by 2,& ...
The Enzymic Activity of the Outer Shell of
... aerobes and which, in contrast to the preparations from lactobacilli, contained enzymes associated with oxygen utilization mediated by the cytochrome hydrogen transport system. The bound ATPase had an optimum at pH 6.0 and was not markedly inhibited by ouabain or oligomycin: it was stimulated by 2,& ...
... aerobes and which, in contrast to the preparations from lactobacilli, contained enzymes associated with oxygen utilization mediated by the cytochrome hydrogen transport system. The bound ATPase had an optimum at pH 6.0 and was not markedly inhibited by ouabain or oligomycin: it was stimulated by 2,& ...
SLG MOCK MIDTERM – FOR PRACTICE ONLY
... 5. Why is the digestion of cellulose not possible for humans? A) Humans do not have enzymes that can hydrolyze the β glycosidic linkages of cellulose. B) The monomer of cellulose is galactose. C) Humans do not have enzymes that can hydrolyze the α glycosidic linkages of cellulose. D) Humans do not ...
... 5. Why is the digestion of cellulose not possible for humans? A) Humans do not have enzymes that can hydrolyze the β glycosidic linkages of cellulose. B) The monomer of cellulose is galactose. C) Humans do not have enzymes that can hydrolyze the α glycosidic linkages of cellulose. D) Humans do not ...
Biosc_48_Chapter_5_lecture
... H+ from the mitochondrial matrix to the space between the inner and outer membranes. b. This sets up a huge concentration gradient of H+ between the membranes. c. H+ can only move through the inner membrane through structures called respiratory assemblies d. Movement of H+ across the membrane provid ...
... H+ from the mitochondrial matrix to the space between the inner and outer membranes. b. This sets up a huge concentration gradient of H+ between the membranes. c. H+ can only move through the inner membrane through structures called respiratory assemblies d. Movement of H+ across the membrane provid ...
Ch 9 and 11 Review Slides
... • fermentation: partial degradation of sugars in the absence of oxygen. • cellular respiration: uses oxygen to complete the breakdown of many organic molecules. • more efficient and widespread ...
... • fermentation: partial degradation of sugars in the absence of oxygen. • cellular respiration: uses oxygen to complete the breakdown of many organic molecules. • more efficient and widespread ...
PDF
... Conversion of glucose to lactic acid is stoichiometrically equivalent to ethanol formation with respect to ATP formation from substrate-level phosphorylation, redox equivalents and product yield. However, anaerobic growth cannot be sustained in homolactate fermenting Saccharomyces cerevisiae. ATP-de ...
... Conversion of glucose to lactic acid is stoichiometrically equivalent to ethanol formation with respect to ATP formation from substrate-level phosphorylation, redox equivalents and product yield. However, anaerobic growth cannot be sustained in homolactate fermenting Saccharomyces cerevisiae. ATP-de ...
Chapter 2 - Water - Technicalsymposium
... Nucleophiles are negatively charged or have unshared pairs of electrons --> attack electrophiles during substitution or addition reactions. Examples of nucleophiles: oxygen, nitrogen, sulfur, carbon, water (weak). Important in condensation reactions, where hydrolysis reactions are favored. e.g. prot ...
... Nucleophiles are negatively charged or have unshared pairs of electrons --> attack electrophiles during substitution or addition reactions. Examples of nucleophiles: oxygen, nitrogen, sulfur, carbon, water (weak). Important in condensation reactions, where hydrolysis reactions are favored. e.g. prot ...
Content Display : Unit 2 - Energy Metabolism : Lesson 1
... form it is called adenosine monophosphate, or AMP), and sometimes two phosphates (adenosine diphosphate, ADP). But when adenosine has three phosphates attached (ATP), it exists in its highest energy form. In fact, ATP is often referred to as a “high-energy phosphate compound,” and the bond that join ...
... form it is called adenosine monophosphate, or AMP), and sometimes two phosphates (adenosine diphosphate, ADP). But when adenosine has three phosphates attached (ATP), it exists in its highest energy form. In fact, ATP is often referred to as a “high-energy phosphate compound,” and the bond that join ...
Chapter 14 - Electron Transport and Oxidative Phosphorylation 14.4
... Uncouplers • Uncouplers stimulate the oxidation of substrates in the absence of ADP • Uncouplers are lipid-soluble weak acids • Both acidic and basic forms can cross the inner mitochondrial membrane • Uncouplers deplete any proton gradient by transporting protons across the membrane Prentice Hall c ...
... Uncouplers • Uncouplers stimulate the oxidation of substrates in the absence of ADP • Uncouplers are lipid-soluble weak acids • Both acidic and basic forms can cross the inner mitochondrial membrane • Uncouplers deplete any proton gradient by transporting protons across the membrane Prentice Hall c ...
the Citric Acid cycle
... Carbohydrates, most notably glucose, are processed by glycolysis into pyruvate. Under aerobic conditions, this is transported into the mitochondria by a specific carrier protein embedded in the mitochondrial membrane and is then oxidatively decarboxylated by the pyruvate dehydrogenase complex to for ...
... Carbohydrates, most notably glucose, are processed by glycolysis into pyruvate. Under aerobic conditions, this is transported into the mitochondria by a specific carrier protein embedded in the mitochondrial membrane and is then oxidatively decarboxylated by the pyruvate dehydrogenase complex to for ...
Chapter 26 Outline Assimilation of Inorganic Nitrogen
... involved measuring nitrogen balance. • Excrete less nitrogen than consumed—positive nitrogen balance (in growth) • Excrete more nitrogen than consumed—negative nitrogen balance (starvation) • If an essential amino acid is omitted from diet, get negative nitrogen balance no matter how much is consume ...
... involved measuring nitrogen balance. • Excrete less nitrogen than consumed—positive nitrogen balance (in growth) • Excrete more nitrogen than consumed—negative nitrogen balance (starvation) • If an essential amino acid is omitted from diet, get negative nitrogen balance no matter how much is consume ...
Glycogen Metabolism Gluconeogenesis
... • In the “resting” state, Gα is bound to the Gβ-Gγ dimer. Gα contains the nucleotide binding site, holding GDP in the inactive form, and is the “warhead” of the G protein. At least 20 different forms of Ga exist in mammalian cells. • Binding of the extracellular signal by the GPCR causes it to under ...
... • In the “resting” state, Gα is bound to the Gβ-Gγ dimer. Gα contains the nucleotide binding site, holding GDP in the inactive form, and is the “warhead” of the G protein. At least 20 different forms of Ga exist in mammalian cells. • Binding of the extracellular signal by the GPCR causes it to under ...
Handout: Fatty Acid Synthesis
... 28.2 Elongation and Desaturation C16:0 to C18:n – C20:n Elongation occurs with enzymes on the cytosolic face of the ER membrane. This is done by elongases that use malonyl-CoA to add the 2-carbon subunits. Oxidase ...
... 28.2 Elongation and Desaturation C16:0 to C18:n – C20:n Elongation occurs with enzymes on the cytosolic face of the ER membrane. This is done by elongases that use malonyl-CoA to add the 2-carbon subunits. Oxidase ...
Biochemistry - DENTISTRY 2012
... ( you have to memorize this reaction because it requires Vitamin B12 which enters in two reactions only ) - So we can measure Vit. B easily by taking a sample of urine ( if there’s a high amount of methylmalonyl there’s a deficiency in Vitamin B . ( methylmalonyl urea ) -Note : the methylmalonyl is ...
... ( you have to memorize this reaction because it requires Vitamin B12 which enters in two reactions only ) - So we can measure Vit. B easily by taking a sample of urine ( if there’s a high amount of methylmalonyl there’s a deficiency in Vitamin B . ( methylmalonyl urea ) -Note : the methylmalonyl is ...
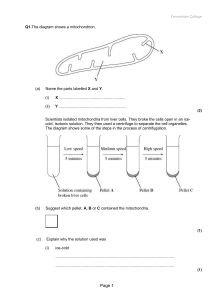
RespirationQuestions.doc - KS3, GCSE and A
... and C, on the electron transport chain in these mitochondria. In each of three experiments, a different inhibitor was added. The table shows the state of the electron carriers, W–Z, after the addition of inhibitor. ...
... and C, on the electron transport chain in these mitochondria. In each of three experiments, a different inhibitor was added. The table shows the state of the electron carriers, W–Z, after the addition of inhibitor. ...
Biol 1406 notes Ch 8 8thed
... ATP (adenosine triphosphate) is a nucleotide triphosphate consisting of the sugar ribose, the nitrogenous base adenine, and a chain of three phosphate groups. ...
... ATP (adenosine triphosphate) is a nucleotide triphosphate consisting of the sugar ribose, the nitrogenous base adenine, and a chain of three phosphate groups. ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.