Pentose Phosphate Pathway (aka Hexose monophosphate shunt)
... • G6PD is the most regulated enzyme: inhibition by NADPH, expression is dependent on insulin thus it is only expressed at high glucose concentration • Since the non-oxidative pathway is reversible, the direction is dependent on the need of the cell for ATP / acetyl CoA (energy / fatty acid synthesis ...
... • G6PD is the most regulated enzyme: inhibition by NADPH, expression is dependent on insulin thus it is only expressed at high glucose concentration • Since the non-oxidative pathway is reversible, the direction is dependent on the need of the cell for ATP / acetyl CoA (energy / fatty acid synthesis ...
P6060Datasheet-Lot0151208
... not exceed 20,000–50,000 units/ml to ensure the suggested rate of autophosphorylation. 2. Substrate Phosphorylation: Mix the substrate with 1X NEBuffer for PK supplemented with ATP. Add the activated CaMKII. Incubate at 30°C. ...
... not exceed 20,000–50,000 units/ml to ensure the suggested rate of autophosphorylation. 2. Substrate Phosphorylation: Mix the substrate with 1X NEBuffer for PK supplemented with ATP. Add the activated CaMKII. Incubate at 30°C. ...
Anatomy and Physiology, 5/e Chapter 27: Nutrition and Metabolism
... they have been digested, absorbed, and circulated to the cells. These two definitions are essential distinctions to be made before moving into the heart of the digestive system physiology. Metabolism can be further broken down into two major processes, catabolism and anabolism. Catabolism breaks foo ...
... they have been digested, absorbed, and circulated to the cells. These two definitions are essential distinctions to be made before moving into the heart of the digestive system physiology. Metabolism can be further broken down into two major processes, catabolism and anabolism. Catabolism breaks foo ...
practice oxidative phosphorylation worksheet11
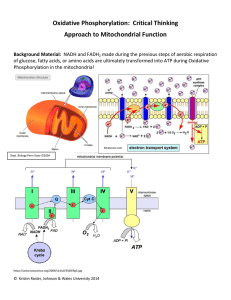
... A series of redox reactions are employed as H+ ions are moved against a concentration gradient via active transport. Protein Complexes I, III, & IV are H+ protein complex ““pump”s” which use the energy harnessed from the oxidation of NADH and FADH2 (electron movement = energy!) in the Electron Trans ...
... A series of redox reactions are employed as H+ ions are moved against a concentration gradient via active transport. Protein Complexes I, III, & IV are H+ protein complex ““pump”s” which use the energy harnessed from the oxidation of NADH and FADH2 (electron movement = energy!) in the Electron Trans ...
video slide - Buena Park High School
... Fermentation and Cellular Respiration Compared • Both fermentation and cellular respiration – Use glycolysis to oxidize glucose and other organic fuels to pyruvate ...
... Fermentation and Cellular Respiration Compared • Both fermentation and cellular respiration – Use glycolysis to oxidize glucose and other organic fuels to pyruvate ...
PYRUVATE OXIDATION, KREBS CYCLE agnes je... 583KB Nov 04
... Quick Review -In glycolysis, the first stage of cellular respiration, glucose, a 6-C chain molecule was broken down into 2 pyruvate molecules in a series of 10 steps. ...
... Quick Review -In glycolysis, the first stage of cellular respiration, glucose, a 6-C chain molecule was broken down into 2 pyruvate molecules in a series of 10 steps. ...
Muscles
... Thin filament – Actin • globular monomer (G-actin) makes a double helix (F-actin) • F-actin has other accessory proteins attached: • tropomyosin (double helix) • troponin C – binds calcium ions • troponin I – inhibits interaction actin-myosin • troponin T – binds to tropomyosin and other troponins ...
... Thin filament – Actin • globular monomer (G-actin) makes a double helix (F-actin) • F-actin has other accessory proteins attached: • tropomyosin (double helix) • troponin C – binds calcium ions • troponin I – inhibits interaction actin-myosin • troponin T – binds to tropomyosin and other troponins ...
C485 Exam I
... 1. (6pts) Which of the following statement about cyclic photophosphorylation are correct? (Circle the correct ones) A) It doesn’t involve NADPH formation B) It uses electrons supplied by photosystem II C) It is activated when NADP+ is limiting D) It does not generate O2 E) It leads to ATP production ...
... 1. (6pts) Which of the following statement about cyclic photophosphorylation are correct? (Circle the correct ones) A) It doesn’t involve NADPH formation B) It uses electrons supplied by photosystem II C) It is activated when NADP+ is limiting D) It does not generate O2 E) It leads to ATP production ...
Introduction to Cell Symbiosis Therapy
... wrong – which lies at the heart of mitochondrial dysfunction – we should look at the two very different forms of energy generation, because this is also key: intramitochondrial, and cytosolic. When ATP is produced in the intracellular fluid (cytosol) via glycolysis, one mole of glucose generates a n ...
... wrong – which lies at the heart of mitochondrial dysfunction – we should look at the two very different forms of energy generation, because this is also key: intramitochondrial, and cytosolic. When ATP is produced in the intracellular fluid (cytosol) via glycolysis, one mole of glucose generates a n ...
Document
... In Stage 1, the digestion of carbohydrates Begins in the mouth where salivary amylase breaks down polysaccharides to smaller polysaccharides (dextrins), maltose, and some glucose. Continues in the small intestine where pancreatic amylase hydrolyzes dextrins to maltose and glucose. Hydrolyzes m ...
... In Stage 1, the digestion of carbohydrates Begins in the mouth where salivary amylase breaks down polysaccharides to smaller polysaccharides (dextrins), maltose, and some glucose. Continues in the small intestine where pancreatic amylase hydrolyzes dextrins to maltose and glucose. Hydrolyzes m ...
free energy - Thunderbird High School
... used to drive an endergonic reaction • Overall, the coupled reactions are exergonic Without looking back at your notes, give the example of each type of work that you used in your previous notes. chemical transport mechanical ...
... used to drive an endergonic reaction • Overall, the coupled reactions are exergonic Without looking back at your notes, give the example of each type of work that you used in your previous notes. chemical transport mechanical ...
pdf file
... (10 nmol/mg protein) (Fig. 4A). AEA had, on the other hand, no effect on the oligomycin sensitive ATP hydrolase activity (Fig. 4B). The ability of AEA to inhibit the ATP synthase activity without affecting the hydrolase activity was further confirmed by the experiment reported in Fig. 5. It is shown ...
... (10 nmol/mg protein) (Fig. 4A). AEA had, on the other hand, no effect on the oligomycin sensitive ATP hydrolase activity (Fig. 4B). The ability of AEA to inhibit the ATP synthase activity without affecting the hydrolase activity was further confirmed by the experiment reported in Fig. 5. It is shown ...
Metabolism
... More accurate model of enzyme action 3-D structure of enzyme fits substrate substrate binding cause enzyme to change shape leading to a tighter fit ...
... More accurate model of enzyme action 3-D structure of enzyme fits substrate substrate binding cause enzyme to change shape leading to a tighter fit ...
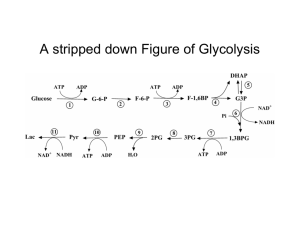
Figure 17-3 Degradation of glucose via the glycolytic pathway.
... The PK bypass uses OAA, which must be generated in the mitochondria. OAA cannot be transported out so it must be converted to PEP or malate (or Asp but lets ignore that). Gluconeogenesis requires NADH so reducing equivalents must be generated for that purpose; cytoplasmic [NADH]/[NAD] is very low. ...
... The PK bypass uses OAA, which must be generated in the mitochondria. OAA cannot be transported out so it must be converted to PEP or malate (or Asp but lets ignore that). Gluconeogenesis requires NADH so reducing equivalents must be generated for that purpose; cytoplasmic [NADH]/[NAD] is very low. ...
chapter 8 notes - 8.4 and 8.5 - APBio09-10
... come together in the proper orientation b. Enzyme stretches the substrate toward transition state form i. Stretches and bends chemical bonds that must be broken in reaction ii. Ea is directly related to the difficulty of breaking the substrate’s bonds iii. Distorting the bonds helps the substrate ap ...
... come together in the proper orientation b. Enzyme stretches the substrate toward transition state form i. Stretches and bends chemical bonds that must be broken in reaction ii. Ea is directly related to the difficulty of breaking the substrate’s bonds iii. Distorting the bonds helps the substrate ap ...
200 µmol /L is far too low a concentration of ammonium to affect
... The effect of forming glutamate from ketoglutarate is to deplete the mitochondrial pool of ketoglutarate, which is a key intermediate in the citric acid cycle. As a result, the rate of citric acid cycle activity falls, so reducing very considerably the rate of formation of ATP. It is this lack of AT ...
... The effect of forming glutamate from ketoglutarate is to deplete the mitochondrial pool of ketoglutarate, which is a key intermediate in the citric acid cycle. As a result, the rate of citric acid cycle activity falls, so reducing very considerably the rate of formation of ATP. It is this lack of AT ...
Cellular Respiration and Fermentation
... You can think of the complete oxidation of glucose via cellular respiration as a set of four interconnected processes that together convert the chemical energy in glucose to chemical energy in ATP. Each of the four processes consists of a distinctive starting molecule, a series of chemical reactions ...
... You can think of the complete oxidation of glucose via cellular respiration as a set of four interconnected processes that together convert the chemical energy in glucose to chemical energy in ATP. Each of the four processes consists of a distinctive starting molecule, a series of chemical reactions ...
How did LUCA make a living?
... and nickel at their centres. The crystal structures of these clusters are essentially those of minerals found in hydrothermal vents, such as greigite,(6,7) to the point that one could refer to the acetyl CoA pathway as having ‘rocky roots’.(6) During the early phases of Earth’s history, >3.8 Gya ago ...
... and nickel at their centres. The crystal structures of these clusters are essentially those of minerals found in hydrothermal vents, such as greigite,(6,7) to the point that one could refer to the acetyl CoA pathway as having ‘rocky roots’.(6) During the early phases of Earth’s history, >3.8 Gya ago ...
Anaerobic-and-Aerobic
... Aerobic respiration, which takes place in the presence of oxygen, evolved after oxygen was added to Earth’s atmosphere. This type of respiration is useful today because the atmosphere is now 21% oxygen. However, some anaerobic organisms that evolved before the atmosphere contained oxygen have surviv ...
... Aerobic respiration, which takes place in the presence of oxygen, evolved after oxygen was added to Earth’s atmosphere. This type of respiration is useful today because the atmosphere is now 21% oxygen. However, some anaerobic organisms that evolved before the atmosphere contained oxygen have surviv ...
Chapter 20 Specific Catabolic Pathways: Carbohydrate, Lipid, and
... Reactions of Pyruvate A key to understanding the biochemical logic behind two of these reactions of pyruvate is to recognize that glycolysis needs a continuing supply of NAD+. • if no oxygen is present to reoxidize NADH to NAD+, then another way must be found to reoxidize. ...
... Reactions of Pyruvate A key to understanding the biochemical logic behind two of these reactions of pyruvate is to recognize that glycolysis needs a continuing supply of NAD+. • if no oxygen is present to reoxidize NADH to NAD+, then another way must be found to reoxidize. ...
12_Lecture
... • Fructose that enters a cell flows from reaction 5 to 10. • Fructose uptake by the cells is not regulated by insulin: all fructose in the bloodstream is forced into catabolism. • Glycolysis is regulated at step 3. The triose products created in the liver provide an excess of reactants that create e ...
... • Fructose that enters a cell flows from reaction 5 to 10. • Fructose uptake by the cells is not regulated by insulin: all fructose in the bloodstream is forced into catabolism. • Glycolysis is regulated at step 3. The triose products created in the liver provide an excess of reactants that create e ...
Metabolism of Carbohydrates
... Adenosine Tri-Phosphate (ATP) Link between energy releasing and energy requiring mechanisms ...
... Adenosine Tri-Phosphate (ATP) Link between energy releasing and energy requiring mechanisms ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.