Review Packet CORRECT
... a. What goes into the Krebs cycle? Acetyl CoA, NAD+, FADH+, ADP + P b. What comes out of the Krebs cycle? CO2, 6 NADH, 2 FADH2, ATP c. What is another name for the Krebs cycle? Citric Acid Cycle ...
... a. What goes into the Krebs cycle? Acetyl CoA, NAD+, FADH+, ADP + P b. What comes out of the Krebs cycle? CO2, 6 NADH, 2 FADH2, ATP c. What is another name for the Krebs cycle? Citric Acid Cycle ...
1 BIOCHEMISTRY All organic compounds must contain and Are the
... b) One enzyme can facilitate the reaction of many different substrates c) Enzymes are not required for spontaneous reactions d) Not all catalysts are enzymes e) The active site of an enzyme will denature at high temperatures 4) What are the components of nucleotides? a) Glycerols, fatty acids and ph ...
... b) One enzyme can facilitate the reaction of many different substrates c) Enzymes are not required for spontaneous reactions d) Not all catalysts are enzymes e) The active site of an enzyme will denature at high temperatures 4) What are the components of nucleotides? a) Glycerols, fatty acids and ph ...
BIOB111 - Tutorial activity for Session 21
... Answer these questions a. Where in the cell does the citric acid cycle (Krebs cycle) occur b. ...
... Answer these questions a. Where in the cell does the citric acid cycle (Krebs cycle) occur b. ...
Problem Set 9 Key
... 1. Describe the process of delivering amino acids to the liver from: a. Dietary proteins Gastrin Hormone is secreted by gastric mucosal cells which signals the release of HCl and Pepsinogen (pepsin zymogen) by gastric glands. The low pH triggesr Secretin release, which stimulates pancrease to releas ...
... 1. Describe the process of delivering amino acids to the liver from: a. Dietary proteins Gastrin Hormone is secreted by gastric mucosal cells which signals the release of HCl and Pepsinogen (pepsin zymogen) by gastric glands. The low pH triggesr Secretin release, which stimulates pancrease to releas ...
chapter 4 pptol
... Mechanical energy Sound Chemical energy ATP Molecules Each ATP molecule has three parts: When the bond is broken, energy is An adenine molecule transferred A ribose molecule When the bond is broken, ATP Three phosphate molecules in a becomes ADP chain ADP becomes ATP through Third phosphate attached ...
... Mechanical energy Sound Chemical energy ATP Molecules Each ATP molecule has three parts: When the bond is broken, energy is An adenine molecule transferred A ribose molecule When the bond is broken, ATP Three phosphate molecules in a becomes ADP chain ADP becomes ATP through Third phosphate attached ...
Name: Date: Concept Check Questions Chapter 8 (orange) or 6
... exergonic or endergonic? What happens to the energy released from glucose? 2. A key process in metabolism is the transfer of H+ ions across a membrane to create a concentration gradient. In some conditions, H+ ions flow back across the membrane and come to equal concentrations on each side. In which ...
... exergonic or endergonic? What happens to the energy released from glucose? 2. A key process in metabolism is the transfer of H+ ions across a membrane to create a concentration gradient. In some conditions, H+ ions flow back across the membrane and come to equal concentrations on each side. In which ...
acetyl CoA
... • Remember that the citric acid cycle processes two molecules of acetyl CoA for each initial glucose. • Thus, after two turns of the citric acid cycle, the overall yield per glucose molecule is – 2 ATP, – 6 NADH, and – 2 FADH2. ...
... • Remember that the citric acid cycle processes two molecules of acetyl CoA for each initial glucose. • Thus, after two turns of the citric acid cycle, the overall yield per glucose molecule is – 2 ATP, – 6 NADH, and – 2 FADH2. ...
Exam 2
... Figure 10.5 (a) The energy of activation is a barrier that prevents molecules from undergoing otherwise favorable reactions. (b) Enzymes lower the energy of activation barrier, allowing the reaction to proceed. ...
... Figure 10.5 (a) The energy of activation is a barrier that prevents molecules from undergoing otherwise favorable reactions. (b) Enzymes lower the energy of activation barrier, allowing the reaction to proceed. ...
Problem Set 1 - Berkeley MCB
... (C) is activated by the hormone insulin (D) is essential in the conversion of fatty acids to glucose. (E) requires the enzyme hexokinase. ...
... (C) is activated by the hormone insulin (D) is essential in the conversion of fatty acids to glucose. (E) requires the enzyme hexokinase. ...
Mitochondria
... – hundreds of enzymes – special mitochondrial ribosomes, tRNAs and mRNAs – several copies of the mitochondrial DNA genome ...
... – hundreds of enzymes – special mitochondrial ribosomes, tRNAs and mRNAs – several copies of the mitochondrial DNA genome ...
Ch - Humble ISD
... Catabolism – chemical rxns (usually Hydrolysis reactions) Break down larger food molecules into smaller chem. units; release energy ...
... Catabolism – chemical rxns (usually Hydrolysis reactions) Break down larger food molecules into smaller chem. units; release energy ...
Chapter 8 Notes – Energy and Metabolism
... transferred to the outside of the membrane by the cytochrome c oxidase complex. The cytochrome oxidase complex then transfers electrons from cytochrome c to oxygen, the terminal electron acceptor and water is formed as the product. The transfer of protons to the intermembrane space generates a proto ...
... transferred to the outside of the membrane by the cytochrome c oxidase complex. The cytochrome oxidase complex then transfers electrons from cytochrome c to oxygen, the terminal electron acceptor and water is formed as the product. The transfer of protons to the intermembrane space generates a proto ...
Unit 3
... breakdown of glucose to pyruvate without the use of oxygen. Pyruvate is then converted into lactic acid, which limits the amount of ATP produced (2 ATP molecules). ...
... breakdown of glucose to pyruvate without the use of oxygen. Pyruvate is then converted into lactic acid, which limits the amount of ATP produced (2 ATP molecules). ...
32Ch09ATP2008 (1)
... Can’t store ATP cellular good energy donor, not good energy storage respiration too reactive transfers Pi too easily only short term energy storage carbohydrates & fats are long term energy storage Whoa! Pass me the glucose (and O2)! AP Biology ...
... Can’t store ATP cellular good energy donor, not good energy storage respiration too reactive transfers Pi too easily only short term energy storage carbohydrates & fats are long term energy storage Whoa! Pass me the glucose (and O2)! AP Biology ...
Sample Question Set 5a
... maximum number of ATPs that can be synthesized, assuming standard conditions and 100% conservation of energy? (ΔG°’ = 30.5 kJ mol-1 for ATP synthesis) 18. When the F1 prtion of the ATP synthase complex is removed from the mitochondrial membrane and studied in solution, it functions as an ATPase, i.e ...
... maximum number of ATPs that can be synthesized, assuming standard conditions and 100% conservation of energy? (ΔG°’ = 30.5 kJ mol-1 for ATP synthesis) 18. When the F1 prtion of the ATP synthase complex is removed from the mitochondrial membrane and studied in solution, it functions as an ATPase, i.e ...
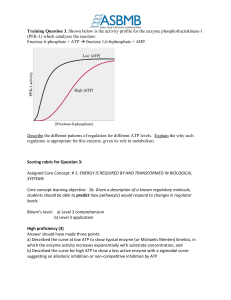
Training Question 3: Rubric
... levels. Bloom’s level: a) Level 2 comprehension b) Level 3 application High proficiency (3) Answer should have made three points: a) Described the curve at low ATP to show typical enzyme (or Michaelis Menten) kinetics, in which the enzyme activity increases exponentially with substrate concentration ...
... levels. Bloom’s level: a) Level 2 comprehension b) Level 3 application High proficiency (3) Answer should have made three points: a) Described the curve at low ATP to show typical enzyme (or Michaelis Menten) kinetics, in which the enzyme activity increases exponentially with substrate concentration ...
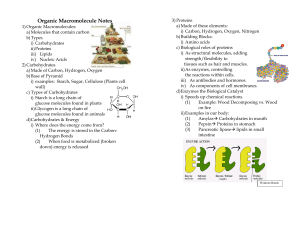
Organic Macromolecule Notes
... a) Made of these elements: i) Carbon, Hydrogen, Oxygen, Nitrogen b) Building Blocks: i) Amino acids c) Biological roles of proteins i) As structural molecules, adding strength/flexibility to tissues such as hair and muscles. ii) As enzymes, controlling the reactions within cells. iii) As antibodies ...
... a) Made of these elements: i) Carbon, Hydrogen, Oxygen, Nitrogen b) Building Blocks: i) Amino acids c) Biological roles of proteins i) As structural molecules, adding strength/flexibility to tissues such as hair and muscles. ii) As enzymes, controlling the reactions within cells. iii) As antibodies ...
Name Answer Key Date Period 3.7 Cell Respiration 1. Define cell
... regenerate oxaloacetate. Decarboxylation will produce 2 CO2 and the redox reactions will produce 3 NADH and 1 FADH2 by transferring electrons from citrate to the electron carriers. ATP will also be produced by substrate-level phosphorylation. For each molecule of glucose that is broken down, the Kre ...
... regenerate oxaloacetate. Decarboxylation will produce 2 CO2 and the redox reactions will produce 3 NADH and 1 FADH2 by transferring electrons from citrate to the electron carriers. ATP will also be produced by substrate-level phosphorylation. For each molecule of glucose that is broken down, the Kre ...
Exam 3
... 16. Where does Krebs cycle occur? A. in the mitochondrial matrix B. in the cytoplasm C. in the chloroplast D. in the inner membrane of the mitochondria 17. Which 2-carbon molecule is produced by the preparatory steps/conversions? A. B. C. D. ...
... 16. Where does Krebs cycle occur? A. in the mitochondrial matrix B. in the cytoplasm C. in the chloroplast D. in the inner membrane of the mitochondria 17. Which 2-carbon molecule is produced by the preparatory steps/conversions? A. B. C. D. ...
9.3 student Fill in notes
... In the second stage, pyruvate either passes through the _________________ or undergoes ___________________ – Fermentation recycles __________ but does not produce _____________. ...
... In the second stage, pyruvate either passes through the _________________ or undergoes ___________________ – Fermentation recycles __________ but does not produce _____________. ...
Cellular Respiration
... release free energy. This free energy is used to pump H+ protons into the inner membrane space of the mitochondria This creates an electro-chemical gradient that is a source of free energy which is used to create ATP! ...
... release free energy. This free energy is used to pump H+ protons into the inner membrane space of the mitochondria This creates an electro-chemical gradient that is a source of free energy which is used to create ATP! ...
2) Where
... • A molecule capable of accepDng one or more electrons from another molecule • Carriers donate these electrons to another molecule during the process of electron transport. • The energy from the electrons ...
... • A molecule capable of accepDng one or more electrons from another molecule • Carriers donate these electrons to another molecule during the process of electron transport. • The energy from the electrons ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.