notes - is234
... Cellular respiration is the process used by humans and most other organisms to release the energy stored in the food they consume. ...
... Cellular respiration is the process used by humans and most other organisms to release the energy stored in the food they consume. ...
Name Date Period Chapter 9: Cellular Respiration: Harvesting
... 3. What is the summary equation for cellular respiration and what is the free energy change in this process? ...
... 3. What is the summary equation for cellular respiration and what is the free energy change in this process? ...
Chapter 8 Summary
... Cells rely on the energy contained in the chemical bonds of ATP. Some ATP is generated by substrate phosphorylation, a process that adds a phosphate group (P i) directly to ADP. However, most ATP is synthesized by oxidative phosphorylation, which involves a series of chemical reactions that make up ...
... Cells rely on the energy contained in the chemical bonds of ATP. Some ATP is generated by substrate phosphorylation, a process that adds a phosphate group (P i) directly to ADP. However, most ATP is synthesized by oxidative phosphorylation, which involves a series of chemical reactions that make up ...
Carbohydrate and sugar structure
... Why is the hydrolysis of ATP energetic? 1. Resonance stabilization of a phosphoanhydride bond is less than that of its hydrolysis products. 2. Electrostatic repulsion between three of four negative charges on the phosphate at neutral pH. DG becomes even lower at higher pH values which produces more ...
... Why is the hydrolysis of ATP energetic? 1. Resonance stabilization of a phosphoanhydride bond is less than that of its hydrolysis products. 2. Electrostatic repulsion between three of four negative charges on the phosphate at neutral pH. DG becomes even lower at higher pH values which produces more ...
Oxidative phosphorylation (mitochondria)
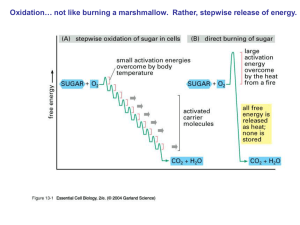
... Oxidation… not like burning a marshmallow. Rather, stepwise release of energy. ...
... Oxidation… not like burning a marshmallow. Rather, stepwise release of energy. ...
Recap: structure of ATP
... ATP is a source of energy Energy is released when ATP spits and forms ADP. The energy from this split is immediately available. A lot of the energy produced by cells ends up as heat (environment) therefore the body needs a continual source of energy. Cell respiration ...
... ATP is a source of energy Energy is released when ATP spits and forms ADP. The energy from this split is immediately available. A lot of the energy produced by cells ends up as heat (environment) therefore the body needs a continual source of energy. Cell respiration ...
Khan Academy 15min cell respiration
... An ATP synthase (EC 3.6.3.14) is a general term for an enzyme that can synthesize adenosine triphosphate (ATP) from adenosine diphosphate (ADP) and inorganic phosphate by using some form of energy. This energy is often in the form of protons moving down an electrochemical gradient, such as from the ...
... An ATP synthase (EC 3.6.3.14) is a general term for an enzyme that can synthesize adenosine triphosphate (ATP) from adenosine diphosphate (ADP) and inorganic phosphate by using some form of energy. This energy is often in the form of protons moving down an electrochemical gradient, such as from the ...
Cellular Respiration
... A carbon is removed, forming CO2 Electrons are removed: NAD+ NADH Coenzyme A joins the 2-carbon molecule, forming Acetyl-Co-A Acetyl-Co-A then adds the 2-carbon acetyl group to a 4-carbon compound (oxaloacetate), forming Citric Acid ...
... A carbon is removed, forming CO2 Electrons are removed: NAD+ NADH Coenzyme A joins the 2-carbon molecule, forming Acetyl-Co-A Acetyl-Co-A then adds the 2-carbon acetyl group to a 4-carbon compound (oxaloacetate), forming Citric Acid ...
Cellular Respiration CPB
... uses H-E e- from Krebs to convert ADP ATP eukaryotes: series of proteins embedded in the inner membrane of mitochondrion prokaryotes: same chain in cell membrane H-E e- move from 1 carrier protein to the next E is used to move H ions across membrane (ATP synthase) every rotation of ATPas ...
... uses H-E e- from Krebs to convert ADP ATP eukaryotes: series of proteins embedded in the inner membrane of mitochondrion prokaryotes: same chain in cell membrane H-E e- move from 1 carrier protein to the next E is used to move H ions across membrane (ATP synthase) every rotation of ATPas ...
glucose, faKy acids, amino acids
... 3 Steps of Cellular Respira4on (each produces some ATP) 1) Glycolysis -‐ spliYng of glucose (2 ATP) (anaerobic -‐ no O2 needed) 2) Citric Acid (Krebs) cycle (2 ATP) (aerobic -‐ ...
... 3 Steps of Cellular Respira4on (each produces some ATP) 1) Glycolysis -‐ spliYng of glucose (2 ATP) (anaerobic -‐ no O2 needed) 2) Citric Acid (Krebs) cycle (2 ATP) (aerobic -‐ ...
Metabolism08
... From Carbohydrate – glucose From Lipids – glycerol and fatty acids From Protein – amino acids ...
... From Carbohydrate – glucose From Lipids – glycerol and fatty acids From Protein – amino acids ...
Homework #4: VERSION 2.1
... you hand it in, you will be asked to evaluate someone else’s homework – the quality of your evaluation will be worth an additional 4 points. Your final grade for this assignment will be determined ...
... you hand it in, you will be asked to evaluate someone else’s homework – the quality of your evaluation will be worth an additional 4 points. Your final grade for this assignment will be determined ...
Energy Yields from Aerobic Respiration: Some Alternatives
... cells of the body. In stage II, these monomers enter cells of the body and are converted into a form that can be completely oxidized. For carbohydrates, glucose is used as a substrate for the glycolysis pathway, the first stage of carbohydrate metabolism. In this pathway, glucose is converted into t ...
... cells of the body. In stage II, these monomers enter cells of the body and are converted into a form that can be completely oxidized. For carbohydrates, glucose is used as a substrate for the glycolysis pathway, the first stage of carbohydrate metabolism. In this pathway, glucose is converted into t ...
Chapter 9 Study Guide
... a. has a stable phosphate bond b. has been formed by the reaction ADP + P → ATP c. has an increased reactivity; it is primed to do work. d. has been oxidized e. will pass its electrons to the electron transport chain. ______16. Which of the following is not true of oxidative Phosphorylation? a. It p ...
... a. has a stable phosphate bond b. has been formed by the reaction ADP + P → ATP c. has an increased reactivity; it is primed to do work. d. has been oxidized e. will pass its electrons to the electron transport chain. ______16. Which of the following is not true of oxidative Phosphorylation? a. It p ...
Macromoleucles Notes
... Cells and their organelles are made up of smaller building blocks called ______________________________. There are 4 basic types of macromolecules. They are: o ________________ o ____________________ o __________________________ o ________________ ______________ Monomers and polymers o Macromo ...
... Cells and their organelles are made up of smaller building blocks called ______________________________. There are 4 basic types of macromolecules. They are: o ________________ o ____________________ o __________________________ o ________________ ______________ Monomers and polymers o Macromo ...
Introduction to Microbiology BIOL 220, Summer Session 1, 1996
... A. It interacts with enzyme at a site other than the active site. B. It typically interferes with many enzymes. C. Its effects are usually irreversible. D. It cannot be converted to products by the enzyme which it inhibits. __A___12. What is the function of enzymes within cells? A. To lower the acti ...
... A. It interacts with enzyme at a site other than the active site. B. It typically interferes with many enzymes. C. Its effects are usually irreversible. D. It cannot be converted to products by the enzyme which it inhibits. __A___12. What is the function of enzymes within cells? A. To lower the acti ...
Cell Respiration
... partial oxidation releases energy that forms a small amount of ATP. • This is an anaerobic process and 2 net ATP are generated. • This ATP is used to start the Kreb’s Cycle. ...
... partial oxidation releases energy that forms a small amount of ATP. • This is an anaerobic process and 2 net ATP are generated. • This ATP is used to start the Kreb’s Cycle. ...
Ans 518_class 4
... • The pathway in which GLUT4 is integrated into the plasma membrane begins with insulin binding to its receptor dimer • The receptor dimer autophosphorylates (intrinsic tyrosine kinase activity) and subsequently activates insulin-responsive substrate-1 (IRS1), which initiates a series of reactions t ...
... • The pathway in which GLUT4 is integrated into the plasma membrane begins with insulin binding to its receptor dimer • The receptor dimer autophosphorylates (intrinsic tyrosine kinase activity) and subsequently activates insulin-responsive substrate-1 (IRS1), which initiates a series of reactions t ...
Chapter 6 Cellular Energy
... electrochemical gradient drives the diffusion of H+ ions through ATP synthase ( enzyme) producing molecules of ATP Oxygen acts as the final electron acceptor, it bonds with 2 H+ ions to create water ...
... electrochemical gradient drives the diffusion of H+ ions through ATP synthase ( enzyme) producing molecules of ATP Oxygen acts as the final electron acceptor, it bonds with 2 H+ ions to create water ...
Lecture 19
... •Isozymes: enzymes that catalyze the same reaction but are encoded by different genes and have different kinetic of regulatory properties. •Lactate dehydrogenase (LDH): type M [skeletal muscle and liver] participates in the reduction of pyruvate to lactate (using NADH) while type H [heart muscle] ca ...
... •Isozymes: enzymes that catalyze the same reaction but are encoded by different genes and have different kinetic of regulatory properties. •Lactate dehydrogenase (LDH): type M [skeletal muscle and liver] participates in the reduction of pyruvate to lactate (using NADH) while type H [heart muscle] ca ...
Metabolism
... electron transport system to yield ATP 2 turns of cycle are completed for each glucose molecule 2 ATP + 6 NADH +2 FADH2 ...
... electron transport system to yield ATP 2 turns of cycle are completed for each glucose molecule 2 ATP + 6 NADH +2 FADH2 ...
Biology STAAR EOC Review Sheets Alief
... shoot system. When one side of a plant does not receive enough light, a hormone that causes growth is produced in the shoot system’s leaves. It is transported to the darker side. As the dark side grows, the plant bends toward the light. ...
... shoot system. When one side of a plant does not receive enough light, a hormone that causes growth is produced in the shoot system’s leaves. It is transported to the darker side. As the dark side grows, the plant bends toward the light. ...
Lecture #4 Date
... • Krebs Cycle: location mitochondrial matrix • Electron Transport Chain location: inner membrane of ...
... • Krebs Cycle: location mitochondrial matrix • Electron Transport Chain location: inner membrane of ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.