* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Ch - Humble ISD
Drug discovery wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Biosynthesis wikipedia , lookup
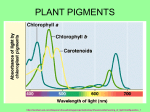
Photosynthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Ch.2 Chemical Basis of Life I. Inorganic Molecules (p. 44) A. Water - ~ 70% of body wt 1. Properties of water (Figs. 2-8, 2-9, 2-10; Table 2-2) a. polarity - allow water to act as effective solvent. (universal solvent) b. tends to ionize substances in solution c. major transport medium d. high specific heat - high heat absorption w/o much change in temp e. High heat of vaporization - absorption of significant amts of heat to chg water from a liquid to a gas B. Electrolytes - substances that can break up, or dissociate, in solution to form ions 1. Acids - release H when in solution a. taste sour b. AKA proton donors c. HCl => H + Cl d. litmus paper will turn red 2. Bases - or alkaline compounds, when dissociated in soln, shift the H /OH balance in favor of OH a. combine or accept H ions - proton acceptor b. taste bitter c. NaOH => Na + OH d. another important base - HCO3 e. litmus paper will turn blue 3. pH scale (Fig. 2-12) - negative log of H conc. a. indicates degree of acidity or alkalinity of solution b. High pH - basic (low H ), pH 8-14 c. Low pH- acidic (high H ), pH 1-6 d. 7 - neutral e. pH: scale 1-14 4. Buffers - minimize changes in the concentration of H and OH to maintain constant pH in body 5. Salts (Tables 2-1, 2-3)-compd that result from chem. rxn of acid and base a. neutralization reaction: HCl + NaOH => NaCl + H2O II. Metabolism (Fig. 2-25) – all of the chemical reactions that occur in body cells A. Catabolism – chemical rxns (usually Hydrolysis reactions) Break down larger food molecules into smaller chem. units; release energy B. Anabolism - chemical rxns (usually dehydration reactions) 1. Build larger and more complex molecules; require energy 2. ex.- Proteins (Table 2-5)- contains C, O, H, N a. most abundant organic molecule in body b. Draw Structure c. peptide bonds – joins amino acids together d. dehydration synthesis- joining of polymers results in loss of water molecule C. Adenosine triphosphate (ATP) (Fig. 2-26) 1. composed of ribose, adenine, & 3 phosphate subunits 2. high energy bonds – energy transferred to newly formed compounds 3. energy stored in ATP used for – muscle contraction & movement, active transport, and biosynthesis