Cell Respiration - Glycolysis PPT
... into two molecules of pyruvate • Glycolysis occurs in the cytoplasm and has two major phases – Energy investment phase – Energy payoff phase ...
... into two molecules of pyruvate • Glycolysis occurs in the cytoplasm and has two major phases – Energy investment phase – Energy payoff phase ...
Pyruvate Dehydrogenase
... • [G-3P] is maintained well below the equilibrium level by being processed through the glycolytic pathway ...
... • [G-3P] is maintained well below the equilibrium level by being processed through the glycolytic pathway ...
Ch 6 Metabolism: Fueling Cell Growth
... Differentiate between, anabolism, and catabolism. Identify the components of an enzyme and describe the mechanism of enzymatic action. List the factors that influence enzymatic activity. Explain what is meant by oxidation–reduction. Describe the chemical reactions of glycolysis. Explain the products ...
... Differentiate between, anabolism, and catabolism. Identify the components of an enzyme and describe the mechanism of enzymatic action. List the factors that influence enzymatic activity. Explain what is meant by oxidation–reduction. Describe the chemical reactions of glycolysis. Explain the products ...
Cellular Respiration Power Point
... • In alcoholic fermentation, pyruvic acid (pyruvate) is converted to CO2 and ethanol – This recycles NAD+ to keep glycolysis working ...
... • In alcoholic fermentation, pyruvic acid (pyruvate) is converted to CO2 and ethanol – This recycles NAD+ to keep glycolysis working ...
enzymes - onlinebiosurgery
... • enzymes are biological catalysts controlling the chemical reactions that take place in all our body cells • they speed up reactions but do not get used up themselves • they are protein molecules made up of long chains of amino acids • they are folded to produce a special shape vital for their func ...
... • enzymes are biological catalysts controlling the chemical reactions that take place in all our body cells • they speed up reactions but do not get used up themselves • they are protein molecules made up of long chains of amino acids • they are folded to produce a special shape vital for their func ...
Ch 6 Metabolism: Fueling Cell Growth
... Differentiate between, anabolism, and catabolism. Identify the components of an enzyme and describe the mechanism of enzymatic action. List the factors that influence enzymatic activity. Explain what is meant by oxidation–reduction. Describe the chemical reactions of glycolysis. Explain the products ...
... Differentiate between, anabolism, and catabolism. Identify the components of an enzyme and describe the mechanism of enzymatic action. List the factors that influence enzymatic activity. Explain what is meant by oxidation–reduction. Describe the chemical reactions of glycolysis. Explain the products ...
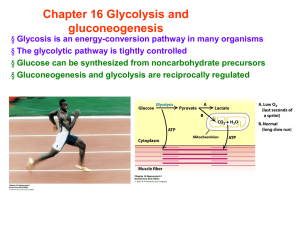
Chapter 16 Glycolysis and gluconeogenesis
... Chapter 16 Glycolysis and gluconeogenesis § Glycosis is an energy-conversion pathway in many organisms § The glycolytic pathway is tightly controlled § Glucose can be synthesized from noncarbohydrate precursors § Gluconeogenesis and glycolysis are reciprocally regulated ...
... Chapter 16 Glycolysis and gluconeogenesis § Glycosis is an energy-conversion pathway in many organisms § The glycolytic pathway is tightly controlled § Glucose can be synthesized from noncarbohydrate precursors § Gluconeogenesis and glycolysis are reciprocally regulated ...
Complete breakdown of Glucose:
... DO NOT COPY! This figure won’t be on the exam, I promise! But you still need to know what goes in and what comes out ...
... DO NOT COPY! This figure won’t be on the exam, I promise! But you still need to know what goes in and what comes out ...
File - myrnafoxsciencespot
... ** it takes two ATP to transfer the electrons from NADH produced by glycolysis (in the cytoplasm) through the mitochondrial membrane into the matrix. Consequently, the total ATP produced by the aerobic respiration of one glucose is 36. Even the total of 36 ATP per glucose is an average because the c ...
... ** it takes two ATP to transfer the electrons from NADH produced by glycolysis (in the cytoplasm) through the mitochondrial membrane into the matrix. Consequently, the total ATP produced by the aerobic respiration of one glucose is 36. Even the total of 36 ATP per glucose is an average because the c ...
sample
... The electron transport chain (ETC) The electron transport chain involves a chain of electron carriers located on the inner mitochondrial membrane (cristae). The cristae have a large surface area so there are more electron carriers, which increases ATP synthesis. The reduced coenzymes, NADH 2 and FAD ...
... The electron transport chain (ETC) The electron transport chain involves a chain of electron carriers located on the inner mitochondrial membrane (cristae). The cristae have a large surface area so there are more electron carriers, which increases ATP synthesis. The reduced coenzymes, NADH 2 and FAD ...
Enzymes - WordPress.com
... • Enzymes are protein catalysts that increase the velocity of a chemical reaction, and are not consumed during the reaction they catalyze. [Note: Some types of RNA can act like enzymes, usually catalyzing the cleavage and synthesis of phosphodiester bonds. RNAs with catalytic activity are called rib ...
... • Enzymes are protein catalysts that increase the velocity of a chemical reaction, and are not consumed during the reaction they catalyze. [Note: Some types of RNA can act like enzymes, usually catalyzing the cleavage and synthesis of phosphodiester bonds. RNAs with catalytic activity are called rib ...
Chapter 1 Notes
... compounds dioxide C6H12O6 + 6O2 6CO2 + 6H2O + Energy 1 glucose = -686 kcals ...
... compounds dioxide C6H12O6 + 6O2 6CO2 + 6H2O + Energy 1 glucose = -686 kcals ...
MEMBRANE-BOUND ELECTRON TRANSFER AND ATP …
... diffuses rapidly within the IMM. Electrons are carried from Complex III to Complex IV by cytochrome c, a small hydrophilic peripheral membrane protein located on the cytosolic or P side of the IMM. Complex II (Succinate-UQ oxidoreductase) is membrane bound and contains the FADH2 as a prosthetic grou ...
... diffuses rapidly within the IMM. Electrons are carried from Complex III to Complex IV by cytochrome c, a small hydrophilic peripheral membrane protein located on the cytosolic or P side of the IMM. Complex II (Succinate-UQ oxidoreductase) is membrane bound and contains the FADH2 as a prosthetic grou ...
You Light Up My Life - Hillsborough Community College
... • When ATP levels rise high enough, glucose6-phosphate is diverted into glycogen synthesis (mainly in liver and muscle) • Glycogen is the main storage polysaccharide in animals ...
... • When ATP levels rise high enough, glucose6-phosphate is diverted into glycogen synthesis (mainly in liver and muscle) • Glycogen is the main storage polysaccharide in animals ...
Chapter 1 Notes
... compounds dioxide C6H12O6 + 6O2 6CO2 + 6H2O + Energy 1 glucose = -686 kcals ...
... compounds dioxide C6H12O6 + 6O2 6CO2 + 6H2O + Energy 1 glucose = -686 kcals ...
bme-biochem-5-1-atp-adp-cycle-kh-6
... ATP PRODUCTION FROM CARBOHYDRATES Electron Transport Chain A series of Oxidative Phosphorylation reactions Oxidation = the removal of electrons from a molecule and results in a decrease in the energy content of the molecule. Because most biological reactions involve the loss of hydrogen atoms, they ...
... ATP PRODUCTION FROM CARBOHYDRATES Electron Transport Chain A series of Oxidative Phosphorylation reactions Oxidation = the removal of electrons from a molecule and results in a decrease in the energy content of the molecule. Because most biological reactions involve the loss of hydrogen atoms, they ...
4.5 Cellular Respiration in Detail
... • The Krebs cycle produces energy-carrying molecules. – NADH and FADH2 are made – intermediate molecule with CoA enters Krebs cycle – citric acid (six-carbon molecule) is formed – citric acid is broken down, carbon dioxide is released, and NADH is made – five-carbon molecule is broken down, carbon d ...
... • The Krebs cycle produces energy-carrying molecules. – NADH and FADH2 are made – intermediate molecule with CoA enters Krebs cycle – citric acid (six-carbon molecule) is formed – citric acid is broken down, carbon dioxide is released, and NADH is made – five-carbon molecule is broken down, carbon d ...
4.5 Cellular Respiration in Detail KEY CONCEPT main stages.
... • The Krebs cycle produces energy-carrying molecules. – NADH and FADH2 are made – intermediate molecule with CoA enters Krebs cycle – citric acid (six-carbon molecule) is formed – citric acid is broken down, carbon dioxide is released, and NADH is made – five-carbon molecule is broken down, carbon d ...
... • The Krebs cycle produces energy-carrying molecules. – NADH and FADH2 are made – intermediate molecule with CoA enters Krebs cycle – citric acid (six-carbon molecule) is formed – citric acid is broken down, carbon dioxide is released, and NADH is made – five-carbon molecule is broken down, carbon d ...
Notes
... the citric acid cycle. As soon as acetyl-CoA is formed, then the acetic acid component (2 carbon compound) can combine with oxaloacetic acid (4 carbon compounds) to make a molecule of citric acid (6 carbon compounds). Co-enzyme A acts only as a transporter of acetic acid. The formation of citric aci ...
... the citric acid cycle. As soon as acetyl-CoA is formed, then the acetic acid component (2 carbon compound) can combine with oxaloacetic acid (4 carbon compounds) to make a molecule of citric acid (6 carbon compounds). Co-enzyme A acts only as a transporter of acetic acid. The formation of citric aci ...
Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency
... deficiency is also known as "favism," since G6PD deficient individuals are also sometimes allergic to fava beans. G6PD deficiency is an allelic abnormality which is inherited in an X-linked recessive fashion ...
... deficiency is also known as "favism," since G6PD deficient individuals are also sometimes allergic to fava beans. G6PD deficiency is an allelic abnormality which is inherited in an X-linked recessive fashion ...
The TCA cycle
... Energy is produced and trapped as ATP by oxidative phosphorylation. Energy is also produced during the TCA cycle in the form of GTP (which is formally equivalent to ATP). Energy use in man At rest we will consume half our body weight in ATP per day! Of course we cannot store this amount of ATP. As w ...
... Energy is produced and trapped as ATP by oxidative phosphorylation. Energy is also produced during the TCA cycle in the form of GTP (which is formally equivalent to ATP). Energy use in man At rest we will consume half our body weight in ATP per day! Of course we cannot store this amount of ATP. As w ...
Substrate and oxidative phosphorylation
... primarily and firstly in the cytoplasm (in glycolysis) under both aerobic and anaerobic conditions. ...
... primarily and firstly in the cytoplasm (in glycolysis) under both aerobic and anaerobic conditions. ...
the Citric Acid cycle
... present in the inner mitochondrial membrane. The cycle is also an important source of precursors for the building blocks of many other molecules. Fuel molecules can be oxidised (lose electrons). The role of the citric acid cycle is to oxidise an acetyl group to two molecules of CO2, thereby gene ...
... present in the inner mitochondrial membrane. The cycle is also an important source of precursors for the building blocks of many other molecules. Fuel molecules can be oxidised (lose electrons). The role of the citric acid cycle is to oxidise an acetyl group to two molecules of CO2, thereby gene ...
09_Lecture_Presentation
... chemical reactions releases energy stored in organic molecules • This released energy is ultimately used to synthesize ATP ...
... chemical reactions releases energy stored in organic molecules • This released energy is ultimately used to synthesize ATP ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.