J. Anim. Sci. Vol. 80, Suppl. 1/J. Dairy Sci. Vol. 85, Suppl. 1 414 Use
... random use of enzymes on feeds, without consideration for specific situations and substrate targets, will only discourage or delay on-farm adoption of enzyme technology. Research is needed to understand the mode of action of feed enzymes so that efficacy can be assured. While much progress has been ...
... random use of enzymes on feeds, without consideration for specific situations and substrate targets, will only discourage or delay on-farm adoption of enzyme technology. Research is needed to understand the mode of action of feed enzymes so that efficacy can be assured. While much progress has been ...
2 Pyruvic Acid
... The ETC is located on the inner membrane of mitochondria An enzyme called ATP synthase forms ATP by attaching a phosphate to ADP ATP synthase is powered by the transfer of e- along a chain protein complexes that form the ETC. The ETC produces 32-34 ATP per glucose Oxygen removes electrons from the f ...
... The ETC is located on the inner membrane of mitochondria An enzyme called ATP synthase forms ATP by attaching a phosphate to ADP ATP synthase is powered by the transfer of e- along a chain protein complexes that form the ETC. The ETC produces 32-34 ATP per glucose Oxygen removes electrons from the f ...
The Point is to Make ATP!
... C6H12O6 CO2 = fuel has been oxidized electrons attracted to more electronegative atoms in biology, the most electronegative atom? ...
... C6H12O6 CO2 = fuel has been oxidized electrons attracted to more electronegative atoms in biology, the most electronegative atom? ...
The Point is to Make ATP!
... C6H12O6 CO2 = fuel has been oxidized electrons attracted to more electronegative atoms in biology, the most electronegative atom? ...
... C6H12O6 CO2 = fuel has been oxidized electrons attracted to more electronegative atoms in biology, the most electronegative atom? ...
as a PDF
... • The electron transport chain creates enough proton-motive force to produce a maximum of three ATPs for each electron pair that travels from NADH to oxygen. The average yield is actually between two and three ATPs per NADH (2.7). • FADH2 produced during the Krebs Cycle is worth a maximum of only tw ...
... • The electron transport chain creates enough proton-motive force to produce a maximum of three ATPs for each electron pair that travels from NADH to oxygen. The average yield is actually between two and three ATPs per NADH (2.7). • FADH2 produced during the Krebs Cycle is worth a maximum of only tw ...
SBI 4UI Test – Metabolic Processes: Cell Respiration
... F3. Aerobic cellular respiration harvests energy from organic compounds without O2. F4. The total chemical potential energy in the reactants of photosynthesis is less than the total chemical potential energy in the products of photosynthesis. T5. An overall goal of cellular respiration is to move hy ...
... F3. Aerobic cellular respiration harvests energy from organic compounds without O2. F4. The total chemical potential energy in the reactants of photosynthesis is less than the total chemical potential energy in the products of photosynthesis. T5. An overall goal of cellular respiration is to move hy ...
Control of metabolism
... oxygen concentration environmental factors • temperature, CO, some antibiotics ...
... oxygen concentration environmental factors • temperature, CO, some antibiotics ...
Enzymes
... vitamins (respectively) are sometimes need for proper enzymatic activity. • Example 1: Iron must be present in the quaternary structure hemoglobin in order for it to pick up oxygen. zinc must be present to break down alcohol (alcohol dehydrogenase) Example 2: The coenzymes make up a part of the acti ...
... vitamins (respectively) are sometimes need for proper enzymatic activity. • Example 1: Iron must be present in the quaternary structure hemoglobin in order for it to pick up oxygen. zinc must be present to break down alcohol (alcohol dehydrogenase) Example 2: The coenzymes make up a part of the acti ...
Cellular Respiration
... breakdown of glucose occurs here (32/34 ATP molecules) O2 is the final electron acceptor ...
... breakdown of glucose occurs here (32/34 ATP molecules) O2 is the final electron acceptor ...
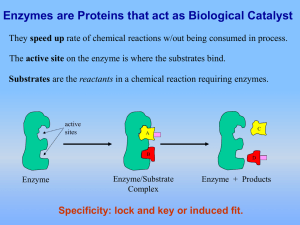
Enzymes
... wililurnto a study of enzyme activity. Take a look at the text below and color the appropriate structures .. Energy must be supplied in order for most cellular chemical reactions to proceed. The initial input of energy required to start a reaction is called the energy of activation. As is shown in t ...
... wililurnto a study of enzyme activity. Take a look at the text below and color the appropriate structures .. Energy must be supplied in order for most cellular chemical reactions to proceed. The initial input of energy required to start a reaction is called the energy of activation. As is shown in t ...
1. Introduction
... intermediate molecular weight organic compounds (metabolites generated by cellular metabolism), and soluble macromolecules (e.g. enzyme proteins, factors, glycogen). Nucleus is coated by the nuclear membrane and contains DNA. The endoplasmic reticulum (ER) is the site of biosynthesis and modificatio ...
... intermediate molecular weight organic compounds (metabolites generated by cellular metabolism), and soluble macromolecules (e.g. enzyme proteins, factors, glycogen). Nucleus is coated by the nuclear membrane and contains DNA. The endoplasmic reticulum (ER) is the site of biosynthesis and modificatio ...
Biology Name_____________________________________
... 9. What is the difference between aerobic and anaerobic respiration? What processes are involved in each type of respiration? ...
... 9. What is the difference between aerobic and anaerobic respiration? What processes are involved in each type of respiration? ...
Workshop3Cellsans
... Both substrate-level phosphorylation and oxidative phosphorylation result in the formation of ATP by the addition of an inorganic phosphate to a molecule of ADP. Both reactions are catalyzed by enzymes that couple the formation of ATP to an exergonic reaction that provides the energy for the synthes ...
... Both substrate-level phosphorylation and oxidative phosphorylation result in the formation of ATP by the addition of an inorganic phosphate to a molecule of ADP. Both reactions are catalyzed by enzymes that couple the formation of ATP to an exergonic reaction that provides the energy for the synthes ...
Many people today are hooked on “fat free” or
... Both substrate-level phosphorylation and oxidative phosphorylation result in the formation of ATP by the addition of an inorganic phosphate to a molecule of ADP. Both reactions are catalyzed by enzymes that couple the formation of ATP to an exergonic reaction that provides the energy for the synthes ...
... Both substrate-level phosphorylation and oxidative phosphorylation result in the formation of ATP by the addition of an inorganic phosphate to a molecule of ADP. Both reactions are catalyzed by enzymes that couple the formation of ATP to an exergonic reaction that provides the energy for the synthes ...
+ Enzyme Inhibitors
... Lethal illness can be caused by the malfunction of just one type of enzyme out of the thousands of types present in our bodies. E.g., the disease phenylketonuria (PKU) results from a mutation of a single amino acid in the enzyme phenylalanine hydroxylase, which catalyzes the first step in the degrad ...
... Lethal illness can be caused by the malfunction of just one type of enzyme out of the thousands of types present in our bodies. E.g., the disease phenylketonuria (PKU) results from a mutation of a single amino acid in the enzyme phenylalanine hydroxylase, which catalyzes the first step in the degrad ...
CH`s 8 - FacStaff Home Page for CBU
... Feedback inhibition is the most common mechanism for control. If ATP concentration begins to drop, respiration speeds up; when there is plenty of ATP, respiration slows down. Control of catabolism is based mainly on regulating the activity of enzymes at strategic points in the catabolic pathway. REV ...
... Feedback inhibition is the most common mechanism for control. If ATP concentration begins to drop, respiration speeds up; when there is plenty of ATP, respiration slows down. Control of catabolism is based mainly on regulating the activity of enzymes at strategic points in the catabolic pathway. REV ...
Topics To Know For Chapter 6
... 12. Know the events of chemiosmosis discussed in class and where does it take place. - thylakoid membrane - ATP synthetase - thylakoid space - electron flow - pH 4 - photosystems I & II - H+ concentration 13. Know what makes the Calvin cycle work or operate. Describe the events taking place in the C ...
... 12. Know the events of chemiosmosis discussed in class and where does it take place. - thylakoid membrane - ATP synthetase - thylakoid space - electron flow - pH 4 - photosystems I & II - H+ concentration 13. Know what makes the Calvin cycle work or operate. Describe the events taking place in the C ...
B. True or False/Edit
... a. Reducing agents donate electrons to another atom or molecule. b. Oxidizing agents accept electrons from another atom or molecule. c. An atom or molecule cannot be both an oxidizing and reducing agent. d. Oxidation and reduction are always coupled reactions. e. All of these statements regarding ox ...
... a. Reducing agents donate electrons to another atom or molecule. b. Oxidizing agents accept electrons from another atom or molecule. c. An atom or molecule cannot be both an oxidizing and reducing agent. d. Oxidation and reduction are always coupled reactions. e. All of these statements regarding ox ...
Chapter 4 - Dr. Dorena Rode
... 1. bioenergetics incorporates these first and second laws 3. the cell's “universal energy carrier” 7. reactions that require energy input 10. oxidizing or reducing ________ 11. different model of the same enzyme 13. compounds mainly derived from water-soluble vitamins 15. inborn error of phenylalani ...
... 1. bioenergetics incorporates these first and second laws 3. the cell's “universal energy carrier” 7. reactions that require energy input 10. oxidizing or reducing ________ 11. different model of the same enzyme 13. compounds mainly derived from water-soluble vitamins 15. inborn error of phenylalani ...
Boardworks Enzyme Inhibitors
... are commonly used in agriculture, the food industry and medicine. Many are enzyme inhibitors. ...
... are commonly used in agriculture, the food industry and medicine. Many are enzyme inhibitors. ...
2. Enzymes
... - are molecules that bind to enzymes and alter catalytic ability. A) Competitive Inhibitors bind to the active site without being acted on, thus reducing reaction rate of true substrate(s). In other cases, the competing molecule is acted on by the enzyme, but again, inhibits reaction with natural su ...
... - are molecules that bind to enzymes and alter catalytic ability. A) Competitive Inhibitors bind to the active site without being acted on, thus reducing reaction rate of true substrate(s). In other cases, the competing molecule is acted on by the enzyme, but again, inhibits reaction with natural su ...
micro notes chpt. 8
... electrons to the electron transport chain for the purpose of generating ATP. c. ...
... electrons to the electron transport chain for the purpose of generating ATP. c. ...
06_Lecture_Presentation - Cornerstone Charter Academy
... In glycolysis, a single molecule of glucose is enzymatically cut in half through a series of steps to produce two molecules of pyruvate – In the process, two molecules of NAD+ are reduced to two molecules of NADH – At the same time, two molecules of ATP are produced by substrate-level phosphorylat ...
... In glycolysis, a single molecule of glucose is enzymatically cut in half through a series of steps to produce two molecules of pyruvate – In the process, two molecules of NAD+ are reduced to two molecules of NADH – At the same time, two molecules of ATP are produced by substrate-level phosphorylat ...
this lecture as PDF here
... • Note potential problem: redox potential for nitrite as electron donor is + 0.42 v., so can easily pass electrons down to oxygen at + 0.82 v., reaction will be spontaneous. Electrons can be passed through an electron transport system, make ATP by chemiosmotic phosphorylation. • BUT --- how to make ...
... • Note potential problem: redox potential for nitrite as electron donor is + 0.42 v., so can easily pass electrons down to oxygen at + 0.82 v., reaction will be spontaneous. Electrons can be passed through an electron transport system, make ATP by chemiosmotic phosphorylation. • BUT --- how to make ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.