Enzymes
... Temperature: cold-->decreased chance of bumping into substrate hot--> good chance of substrate interaction but chance of denaturation at some point pH->change in charge (H+ or OH-) can denature proteins and substrate Examples of pH sensitive enzymes? ...
... Temperature: cold-->decreased chance of bumping into substrate hot--> good chance of substrate interaction but chance of denaturation at some point pH->change in charge (H+ or OH-) can denature proteins and substrate Examples of pH sensitive enzymes? ...
Presentación de PowerPoint
... The quantity kcat/KM is a measure of an enzyme’s catalytic efficiency. There is an upper limit to the value of kcat/KM : It can be not greater than k1; that is, the decomposition of ES to E + P can occur no more frequently than E and S come together to form ES. The most efficient enzymes have kcat/K ...
... The quantity kcat/KM is a measure of an enzyme’s catalytic efficiency. There is an upper limit to the value of kcat/KM : It can be not greater than k1; that is, the decomposition of ES to E + P can occur no more frequently than E and S come together to form ES. The most efficient enzymes have kcat/K ...
Proteins
... simpler parts 4. After completion, the enzyme and the products separate. The enzyme is then ready to react with another substrate. ...
... simpler parts 4. After completion, the enzyme and the products separate. The enzyme is then ready to react with another substrate. ...
Bchm2000_P3 v1 - U of L Class Index
... (4) Ingestion of methanol is a medical emergency. It is often treated by the administration of ethanol, which prevents the dangerous effects of methanol metabolism. In the body, methanol is oxidized to formaldehyde (methanal), a toxic molecule that cannot be further oxidized and that damages protein ...
... (4) Ingestion of methanol is a medical emergency. It is often treated by the administration of ethanol, which prevents the dangerous effects of methanol metabolism. In the body, methanol is oxidized to formaldehyde (methanal), a toxic molecule that cannot be further oxidized and that damages protein ...
CHAPTER-II ENZYMES
... In uncompetitive inhibition, the inhibitor cannot bind to the free enzyme, only to the EScomplex. The EIS-complex thus formed is enzymatically inactive. This type of inhibition is rare, but may occur in multimeric enzymes. Non-competitive inhibition Non-competitive inhibitors can bind to the enzyme ...
... In uncompetitive inhibition, the inhibitor cannot bind to the free enzyme, only to the EScomplex. The EIS-complex thus formed is enzymatically inactive. This type of inhibition is rare, but may occur in multimeric enzymes. Non-competitive inhibition Non-competitive inhibitors can bind to the enzyme ...
Welcome to Jeopardy
... happens to the H+ and the pH? A. The pH and H+ increases B. The pH and H+ decreases C. pH increases, H+ decreases C. pH decreases, H+ increases ...
... happens to the H+ and the pH? A. The pH and H+ increases B. The pH and H+ decreases C. pH increases, H+ decreases C. pH decreases, H+ increases ...
Review Problems #2 (Enzyme Review, Phosphatases
... activity and through regulation of the amount of enzyme that is present. List two general methods of control that affect enzyme activity rather than enzyme amounts. Which of these two methods is employed to control cholesterol biosynthesis? List the control elements that can affect the amount of an ...
... activity and through regulation of the amount of enzyme that is present. List two general methods of control that affect enzyme activity rather than enzyme amounts. Which of these two methods is employed to control cholesterol biosynthesis? List the control elements that can affect the amount of an ...
Enzymes - Mr. hawkins
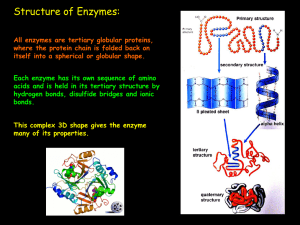
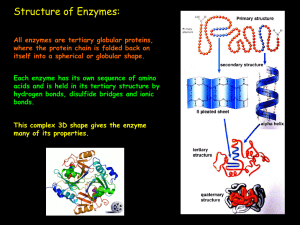
... The Chemical nature of enzymes Enzymes are globular proteins. They have a complex tertiary and quaternary structure in which polypeptides are folded around each other to form a roughly spherical or globular shape. The overall 3D shape of an enzyme molecule is very important: if it is altered, the e ...
... The Chemical nature of enzymes Enzymes are globular proteins. They have a complex tertiary and quaternary structure in which polypeptides are folded around each other to form a roughly spherical or globular shape. The overall 3D shape of an enzyme molecule is very important: if it is altered, the e ...
14-7-SA-V1-S1__enzym..
... b. ATP is required for transport. c. Small organic molecules will diffuse based on molecular weight and solubility in lipids. d. Molecules are transported from low concentration to high concentration. e. Molecules are transported from high concentration to low concentration. ...
... b. ATP is required for transport. c. Small organic molecules will diffuse based on molecular weight and solubility in lipids. d. Molecules are transported from low concentration to high concentration. e. Molecules are transported from high concentration to low concentration. ...
A Crash Course on Enzymology
... • Meat, internal organs, eggs – but these are only rich in proteins. • Proteins are any molecules composed of 40 or more amino acids. • Tons of these in the body and depending on the amino acids making them they behave differently. • Can also be modified to contains other moieties such as metal ions ...
... • Meat, internal organs, eggs – but these are only rich in proteins. • Proteins are any molecules composed of 40 or more amino acids. • Tons of these in the body and depending on the amino acids making them they behave differently. • Can also be modified to contains other moieties such as metal ions ...
CHAPTER 4: Enzyme Structure
... Up to the optimum temperature the rate increases geometrically with temperature (i.e. it's a curve, not a straight line). The rate increases because the enzyme and substrate molecules both have more kinetic energy and so collide more often, and also because more molecules have sufficient energy to o ...
... Up to the optimum temperature the rate increases geometrically with temperature (i.e. it's a curve, not a straight line). The rate increases because the enzyme and substrate molecules both have more kinetic energy and so collide more often, and also because more molecules have sufficient energy to o ...
How Enzymes Work - Manhasset Public Schools
... a) Organic- contains carbon and hydrogen a) Catalysts- affect the rate of chemical reactions ...
... a) Organic- contains carbon and hydrogen a) Catalysts- affect the rate of chemical reactions ...
UNIT 1 PART 1: THE SCIENTIFIC METHOD
... • 2 simple sugars combined to form one molecule. They are table sugars, and also found in fruits and vegetables. Draw a picture of one: • Starches, (glycogen, cellulose-plant cell walls) • String of many simple sugars combined in repeating chains They are used as storage for large sources of energy ...
... • 2 simple sugars combined to form one molecule. They are table sugars, and also found in fruits and vegetables. Draw a picture of one: • Starches, (glycogen, cellulose-plant cell walls) • String of many simple sugars combined in repeating chains They are used as storage for large sources of energy ...
How Enzymes Work - Manhasset Public Schools
... a) Organic- contains carbon and hydrogen a) Catalysts- affect the rate of chemical reactions ...
... a) Organic- contains carbon and hydrogen a) Catalysts- affect the rate of chemical reactions ...
Concept review: Enzyme kinetics
... • A competitive inhibitor ‘competes’ for the same binding site on the enzyme as the substrate. A consequence is that when [S] >> Km the inhibitor will have no effect on the enzyme rate. This type of inhibitor does not change the Vmax but does change the apparent Km. • A noncompetitive inhibitor bind ...
... • A competitive inhibitor ‘competes’ for the same binding site on the enzyme as the substrate. A consequence is that when [S] >> Km the inhibitor will have no effect on the enzyme rate. This type of inhibitor does not change the Vmax but does change the apparent Km. • A noncompetitive inhibitor bind ...
Enzyme structure and function
... This is known as the enzyme’s active site. Each enzyme has a different shape active site which explains why they only interact with specific reactants (which are known as substrates). We call the ‘fit’ between the active site and the substrate complementary. ...
... This is known as the enzyme’s active site. Each enzyme has a different shape active site which explains why they only interact with specific reactants (which are known as substrates). We call the ‘fit’ between the active site and the substrate complementary. ...
Enzyme Lab - Lessons-Worksheets-and-Such
... many normal cellular reactions. If the cells did not break down the hydrogen peroxide, they would be poisoned and die. In this lab, you will study the catalase found in liver cells, potato cells, and apple cells. We can use these enzymes to measure the relative influence of varying several different ...
... many normal cellular reactions. If the cells did not break down the hydrogen peroxide, they would be poisoned and die. In this lab, you will study the catalase found in liver cells, potato cells, and apple cells. We can use these enzymes to measure the relative influence of varying several different ...
a study on cell culture model
... accumulation of glycosaminoglycans (GAG) in lysosomes. These usually fatal disorders are caused by mutations in genes coding for enzymes involved in degradation of GAGs. The deficiencies in activity of appropriate lysosomal enzymes result in various clinical phenotypes which include organomegaly, dy ...
... accumulation of glycosaminoglycans (GAG) in lysosomes. These usually fatal disorders are caused by mutations in genes coding for enzymes involved in degradation of GAGs. The deficiencies in activity of appropriate lysosomal enzymes result in various clinical phenotypes which include organomegaly, dy ...
1.4 Enzymes
... structure and results in inactivation. Extremes of pH can denature the enzyme. The charges on the amino acid side-chains of the enzyme’s active site are affected by free hydrogen ions or hydroxyl ions. In the formation of an enzyme substrate complex the charge on the active site must match those of ...
... structure and results in inactivation. Extremes of pH can denature the enzyme. The charges on the amino acid side-chains of the enzyme’s active site are affected by free hydrogen ions or hydroxyl ions. In the formation of an enzyme substrate complex the charge on the active site must match those of ...
Enzymes revision
... structure and results in inactivation. Extremes of pH can denature the enzyme. The charges on the amino acid side-chains of the enzyme’s active site are affected by free hydrogen ions or hydroxyl ions. In the formation of an enzyme substrate complex the charge on the active site must match those of ...
... structure and results in inactivation. Extremes of pH can denature the enzyme. The charges on the amino acid side-chains of the enzyme’s active site are affected by free hydrogen ions or hydroxyl ions. In the formation of an enzyme substrate complex the charge on the active site must match those of ...
Toothpickase Lab
... Active site = the fold/pocket where an enzyme’s substrate fits into. An enzyme acts only on a specific substrate because only that substrate fits into its active site. Three steps of enzyme activity: 1. A substrate attaches to an enzyme’s active site. 2. The enzyme reduces the activation energy of t ...
... Active site = the fold/pocket where an enzyme’s substrate fits into. An enzyme acts only on a specific substrate because only that substrate fits into its active site. Three steps of enzyme activity: 1. A substrate attaches to an enzyme’s active site. 2. The enzyme reduces the activation energy of t ...
Inorganic/Organic Chemistry Study Guide
... c. Proteins having different levels of organization d. RNA and DNA 7. What is energy of activation? Explain how enzymes enable intracellular chemical reactions by lowering energy of activation? Essays: 1. Enzymes are biological catalysts. a. Relate the chemical structure of an enzyme to its specific ...
... c. Proteins having different levels of organization d. RNA and DNA 7. What is energy of activation? Explain how enzymes enable intracellular chemical reactions by lowering energy of activation? Essays: 1. Enzymes are biological catalysts. a. Relate the chemical structure of an enzyme to its specific ...
6.3 Enzymes and Nucleic Acids ~ powerpoint
... of the enzyme that fits around the substrate. The active site is the keyhole of the lock. • 3. A process called catalysis happens. Catalysis is when the substrate is changed. It could be broken down or combined with another molecule to make something new. • 4. The enzyme lets go. Big idea. When the ...
... of the enzyme that fits around the substrate. The active site is the keyhole of the lock. • 3. A process called catalysis happens. Catalysis is when the substrate is changed. It could be broken down or combined with another molecule to make something new. • 4. The enzyme lets go. Big idea. When the ...
Intro to Metabolism PPT
... What is meant by induced fit? Explain how protein structure is involved in enzyme specificity. Enzymes use a variety of mechanisms to lower activation energy. Describe four of these mechanisms. ...
... What is meant by induced fit? Explain how protein structure is involved in enzyme specificity. Enzymes use a variety of mechanisms to lower activation energy. Describe four of these mechanisms. ...
Enzymes_loop_game
... What is the name of the area on What are enzymes made up of? the enzyme to which the substrate attaches? ...
... What is the name of the area on What are enzymes made up of? the enzyme to which the substrate attaches? ...
Enzyme kinetics

Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or an agonist might inhibit the enzyme.Enzymes are usually protein molecules that manipulate other molecules — the enzymes' substrates. These target molecules bind to an enzyme's active site and are transformed into products through a series of steps known as the enzymatic mechanismE + S <——> ES <——> ES*< ——> EP <——> E + P. These mechanisms can be divided into single-substrate and multiple-substrate mechanisms. Kinetic studies on enzymes that only bind one substrate, such as triosephosphate isomerase, aim to measure the affinity with which the enzyme binds this substrate and the turnover rate. Some other examples of enzymes are phosphofructokinase and hexokinase, both of which are important for cellular respiration (glycolysis).When enzymes bind multiple substrates, such as dihydrofolate reductase (shown right), enzyme kinetics can also show the sequence in which these substrates bind and the sequence in which products are released. An example of enzymes that bind a single substrate and release multiple products are proteases, which cleave one protein substrate into two polypeptide products. Others join two substrates together, such as DNA polymerase linking a nucleotide to DNA. Although these mechanisms are often a complex series of steps, there is typically one rate-determining step that determines the overall kinetics. This rate-determining step may be a chemical reaction or a conformational change of the enzyme or substrates, such as those involved in the release of product(s) from the enzyme.Knowledge of the enzyme's structure is helpful in interpreting kinetic data. For example, the structure can suggest how substrates and products bind during catalysis; what changes occur during the reaction; and even the role of particular amino acid residues in the mechanism. Some enzymes change shape significantly during the mechanism; in such cases, it is helpful to determine the enzyme structure with and without bound substrate analogues that do not undergo the enzymatic reaction.Not all biological catalysts are protein enzymes; RNA-based catalysts such as ribozymes and ribosomes are essential to many cellular functions, such as RNA splicing and translation. The main difference between ribozymes and enzymes is that RNA catalysts are composed of nucleotides, whereas enzymes are composed of amino acids. Ribozymes also perform a more limited set of reactions, although their reaction mechanisms and kinetics can be analysed and classified by the same methods.