Chapter 4 Enzymes and Energy
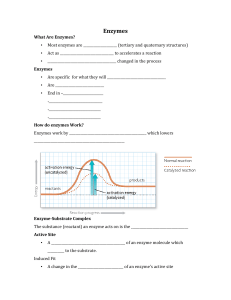
... • Many enzymes work by orienting molecules so that they can better contact each other. • Each type of enzyme has has a highly-ordered, characteristic 3-dimensional shape (conformation). • Ridges, grooves, and pockets lined with specific ...
... • Many enzymes work by orienting molecules so that they can better contact each other. • Each type of enzyme has has a highly-ordered, characteristic 3-dimensional shape (conformation). • Ridges, grooves, and pockets lined with specific ...
Tutorial 1
... Where E donotes chymotrypsin and S is the substrate. P1 and P2 are two products that are generated in two different steps. Suppose the substrate binding equilibrium is fast and the substrate S is in huge excess. Use steady state approximation of the intermediate (ES’) to derive the reaction rate for ...
... Where E donotes chymotrypsin and S is the substrate. P1 and P2 are two products that are generated in two different steps. Suppose the substrate binding equilibrium is fast and the substrate S is in huge excess. Use steady state approximation of the intermediate (ES’) to derive the reaction rate for ...
Unit 2 Biochemistry Chp 8 Metabolism Module
... 1. How are “chemical reactions” important to an organism? Below is a “chemical equation”. 2. If the product is carbonic acid, what are the reactants in this equation? 3. What does the double arrow indicate? 4. What is the biological importance of this equation? Below is the chemical equation for the ...
... 1. How are “chemical reactions” important to an organism? Below is a “chemical equation”. 2. If the product is carbonic acid, what are the reactants in this equation? 3. What does the double arrow indicate? 4. What is the biological importance of this equation? Below is the chemical equation for the ...
Enzyme Puzzle Activity
... and enzymes work together like puzzles. Only one active site of an enzyme will fit in like a puzzle piece with a specific substrate. Thus, they are very specific. Every enzyme has its optimum pH (= pH where it works best). Most enzymes work in almost neutral media. When the temperature increases, mo ...
... and enzymes work together like puzzles. Only one active site of an enzyme will fit in like a puzzle piece with a specific substrate. Thus, they are very specific. Every enzyme has its optimum pH (= pH where it works best). Most enzymes work in almost neutral media. When the temperature increases, mo ...
key
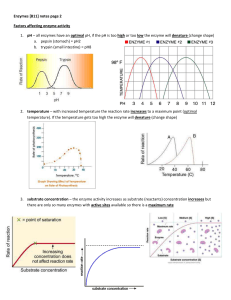
... 2. temperature – with increased temperature the reaction rate increases to a maximum point (optimal temperature). If the temperature gets too high the enzyme will denature (change shape) ...
... 2. temperature – with increased temperature the reaction rate increases to a maximum point (optimal temperature). If the temperature gets too high the enzyme will denature (change shape) ...
chapter 20
... 4. Discuss the difference between the “lock and key” and “induced fit” models of enzyme catalysis. 5. What are some factors that affect enzyme activity? What happens to the rate of the reaction as the available enzyme molecules become saturated with substrate? 6. Discuss differences between competit ...
... 4. Discuss the difference between the “lock and key” and “induced fit” models of enzyme catalysis. 5. What are some factors that affect enzyme activity? What happens to the rate of the reaction as the available enzyme molecules become saturated with substrate? 6. Discuss differences between competit ...
Enzymes - Science Geek
... CA Standard Students know enzymes are proteins that catalyze biochemical reactions without altering the reaction equilibrium and the activities of enzymes depend on the temperature, ionic conditions, and the pH of the surroundings. ...
... CA Standard Students know enzymes are proteins that catalyze biochemical reactions without altering the reaction equilibrium and the activities of enzymes depend on the temperature, ionic conditions, and the pH of the surroundings. ...
Enzyme kinetics

Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or an agonist might inhibit the enzyme.Enzymes are usually protein molecules that manipulate other molecules — the enzymes' substrates. These target molecules bind to an enzyme's active site and are transformed into products through a series of steps known as the enzymatic mechanismE + S <——> ES <——> ES*< ——> EP <——> E + P. These mechanisms can be divided into single-substrate and multiple-substrate mechanisms. Kinetic studies on enzymes that only bind one substrate, such as triosephosphate isomerase, aim to measure the affinity with which the enzyme binds this substrate and the turnover rate. Some other examples of enzymes are phosphofructokinase and hexokinase, both of which are important for cellular respiration (glycolysis).When enzymes bind multiple substrates, such as dihydrofolate reductase (shown right), enzyme kinetics can also show the sequence in which these substrates bind and the sequence in which products are released. An example of enzymes that bind a single substrate and release multiple products are proteases, which cleave one protein substrate into two polypeptide products. Others join two substrates together, such as DNA polymerase linking a nucleotide to DNA. Although these mechanisms are often a complex series of steps, there is typically one rate-determining step that determines the overall kinetics. This rate-determining step may be a chemical reaction or a conformational change of the enzyme or substrates, such as those involved in the release of product(s) from the enzyme.Knowledge of the enzyme's structure is helpful in interpreting kinetic data. For example, the structure can suggest how substrates and products bind during catalysis; what changes occur during the reaction; and even the role of particular amino acid residues in the mechanism. Some enzymes change shape significantly during the mechanism; in such cases, it is helpful to determine the enzyme structure with and without bound substrate analogues that do not undergo the enzymatic reaction.Not all biological catalysts are protein enzymes; RNA-based catalysts such as ribozymes and ribosomes are essential to many cellular functions, such as RNA splicing and translation. The main difference between ribozymes and enzymes is that RNA catalysts are composed of nucleotides, whereas enzymes are composed of amino acids. Ribozymes also perform a more limited set of reactions, although their reaction mechanisms and kinetics can be analysed and classified by the same methods.