A change in temperature affects an enzymatic reaction because
... the enzyme forms bonds with itself instead of the substrate the active site of the enzyme no longer interacts properly with the substrate the enzyme forms abnormal bonds with the substrate ...
... the enzyme forms bonds with itself instead of the substrate the active site of the enzyme no longer interacts properly with the substrate the enzyme forms abnormal bonds with the substrate ...
Active yet responsive approximately equal to the substrate con- centration normally K
... in 1 minute in a 10-ml solution containing 10�9 g of purified penicillinase was measured as a function of the concentration of penicillin. Assume that the concentration of penicillin does not change appreciably during the assay. Penicillin uM ...
... in 1 minute in a 10-ml solution containing 10�9 g of purified penicillinase was measured as a function of the concentration of penicillin. Assume that the concentration of penicillin does not change appreciably during the assay. Penicillin uM ...
Enzyme Quiz # 20 First : Last: 1. Explain how an enzyme speeds up
... 2. Explain how the body is able to activate an enzyme in one part of the digestive tract (i.e stomach) and then denature the enzyme in later parts of the digestive tract ( i.e small intestine ) and at the same time activate other digestive enzymes in the small intestine to help this organ fulfill it ...
... 2. Explain how the body is able to activate an enzyme in one part of the digestive tract (i.e stomach) and then denature the enzyme in later parts of the digestive tract ( i.e small intestine ) and at the same time activate other digestive enzymes in the small intestine to help this organ fulfill it ...
Enzymes - hbwbiology.net
... Regulation of Enzymes – 4 ways of regulating an enzyme: 1. Allosteric enzymes have two kinds of binding sites – an active site for the substrate, and a second binding site for an allosteric ...
... Regulation of Enzymes – 4 ways of regulating an enzyme: 1. Allosteric enzymes have two kinds of binding sites – an active site for the substrate, and a second binding site for an allosteric ...
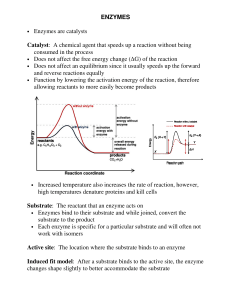
ENZYMES • Enzymes are catalysts Catalyst: A chemical agent that
... absorbed to achieve transition state Active sites can also provide suitable microenvironments for particular reactions (e.g. can provide alternate pH levels depending on the amino acids present in the active site) ...
... absorbed to achieve transition state Active sites can also provide suitable microenvironments for particular reactions (e.g. can provide alternate pH levels depending on the amino acids present in the active site) ...
2. Enzyme activity - Lectures For UG-5
... zero-order kinetics • Enzymes concentrations are always performed in zeroorder kinetics with substrate in sufficient excess to ensure that not more than 20% of the available substrate is converted to product. • Any coenzymes also must be in excess. • NAD or NADH is often convenient as a reagent for ...
... zero-order kinetics • Enzymes concentrations are always performed in zeroorder kinetics with substrate in sufficient excess to ensure that not more than 20% of the available substrate is converted to product. • Any coenzymes also must be in excess. • NAD or NADH is often convenient as a reagent for ...
The link between Darwin and antioxidants from olives
... Eschericia coli cells manipulated to express TMOs are capable of oxidizing a wide range of substituted aromatic and phenolic compounds with high regiospecificity. Despite the resemblance of PEA to the natural substrate, toluene, it was found to be a very poor substrate for the wild-type enzymes. In ...
... Eschericia coli cells manipulated to express TMOs are capable of oxidizing a wide range of substituted aromatic and phenolic compounds with high regiospecificity. Despite the resemblance of PEA to the natural substrate, toluene, it was found to be a very poor substrate for the wild-type enzymes. In ...
Enzymes: The Biological Catalysts
... D. When an enzyme binds with the substrate, the bonded substrate interacts with the enzyme causing it to change shape. This change in shape facilitates the chemical reaction to occur. This is called the induced fit. ...
... D. When an enzyme binds with the substrate, the bonded substrate interacts with the enzyme causing it to change shape. This change in shape facilitates the chemical reaction to occur. This is called the induced fit. ...
Slide 1
... Metabolism is the sum total of all interactions between molecules within cell environments. The chemistry of life is organized into metabolic pathways. Metabolic pathways begin with a specific molecule, which is then altered in a series of defined steps to form a specific product. ...
... Metabolism is the sum total of all interactions between molecules within cell environments. The chemistry of life is organized into metabolic pathways. Metabolic pathways begin with a specific molecule, which is then altered in a series of defined steps to form a specific product. ...
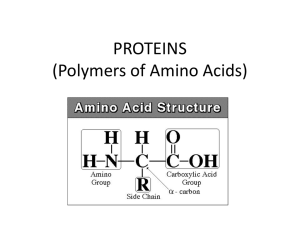
PROTEINS (Polymers of Amino Acids)
... Inhibitors – changes shape of the enzyme without attaching to the active site • Allosteric regulation – attachment of another molecule which changes the shape of the enzyme ...
... Inhibitors – changes shape of the enzyme without attaching to the active site • Allosteric regulation – attachment of another molecule which changes the shape of the enzyme ...
How Do Enzymes Work?
... interactions to the active site The catalyzed reaction takes place at the active site, usually in several steps Two models, lock-and-key vs. induced-fit model Glucose and hexokinase, phosphorylation Improving the binding site for ATP & excluding water (might interfere with the reaction) ...
... interactions to the active site The catalyzed reaction takes place at the active site, usually in several steps Two models, lock-and-key vs. induced-fit model Glucose and hexokinase, phosphorylation Improving the binding site for ATP & excluding water (might interfere with the reaction) ...
Enzymes and How They Work
... 2) Catalysts- chemicals which speed up a reaction without being consumed B. Properties of enzymes: 1) Speed up reactions 2) Can be used over and over, only needed in small amount 3) Decrease amount of energy needed to start a reaction (activation energy) 4) Specific to a substrate based on its shape ...
... 2) Catalysts- chemicals which speed up a reaction without being consumed B. Properties of enzymes: 1) Speed up reactions 2) Can be used over and over, only needed in small amount 3) Decrease amount of energy needed to start a reaction (activation energy) 4) Specific to a substrate based on its shape ...
Chapter 2-ROLE OF ENZYMES
... 22. At low concentrations of competitive inhibitor the reaction rate is still high as few active sites are blocked by the inhibitor leaving the substrates no difficulty in finding free active sites. What happens to the rate of reaction as the concentration of competitive inhibitor increases? ...
... 22. At low concentrations of competitive inhibitor the reaction rate is still high as few active sites are blocked by the inhibitor leaving the substrates no difficulty in finding free active sites. What happens to the rate of reaction as the concentration of competitive inhibitor increases? ...
Chapter 5 Enzymes, Coenzyme and Energy
... The number of molecules of substrate with which a single enzyme can react at a given time (ex. reactions/minute) is known as the turnover number ◦ Can be quite large compared to uncatalyzed reeactions ◦ Can depend on the environment ...
... The number of molecules of substrate with which a single enzyme can react at a given time (ex. reactions/minute) is known as the turnover number ◦ Can be quite large compared to uncatalyzed reeactions ◦ Can depend on the environment ...
Mechanism of enzyme action, kinetic of enzymatic catalysis
... Enzyme is inactive only when bound to inhibitor EI complex is held together by weak, ...
... Enzyme is inactive only when bound to inhibitor EI complex is held together by weak, ...
Enzymes - CynthiaJankowski
... What are Enzymes? • Enzymes are substances called catalysts that speed up chemical rxns by decreasing the activation energy of the rxns. • Enzymes are mostly proteins. • Help maintain homeostasis as rxn in living things would not occur quickly enough to sustain life. • Enzymes usually end in ASE, l ...
... What are Enzymes? • Enzymes are substances called catalysts that speed up chemical rxns by decreasing the activation energy of the rxns. • Enzymes are mostly proteins. • Help maintain homeostasis as rxn in living things would not occur quickly enough to sustain life. • Enzymes usually end in ASE, l ...
AP Biology REVIEW Enzymes MULTIPLE CHOICE QUESTIONS
... Increasing substrate concentration will result in an increased rate of reaction until all available active sites are occupied. At that point, no amount of substrate increase will increase the rate of reaction ...
... Increasing substrate concentration will result in an increased rate of reaction until all available active sites are occupied. At that point, no amount of substrate increase will increase the rate of reaction ...
Enzymes
... (4) The enzyme lets go. Big idea - When the enzyme lets go, it returns to normal, ready to do another reaction. The substrate is no longer the same. The substrate is now called the PRODUCT. ...
... (4) The enzyme lets go. Big idea - When the enzyme lets go, it returns to normal, ready to do another reaction. The substrate is no longer the same. The substrate is now called the PRODUCT. ...
Interfering with enzymes (poisons and drugs)
... • “A non-protein organic molecule that forms a permanent part of a functioning protein molecule.” • E.g. zinc-based prosthetic group in carbonic anhydrase – where have we met this enzyme? ...
... • “A non-protein organic molecule that forms a permanent part of a functioning protein molecule.” • E.g. zinc-based prosthetic group in carbonic anhydrase – where have we met this enzyme? ...
Enzymes in Action Kit – In Brief
... Catabolism is the metabolic process where substances are broken down from complex molecules into simpler molecules. The part of the enzyme that binds the substrate to be acted upon is referred to as the active site. ...
... Catabolism is the metabolic process where substances are broken down from complex molecules into simpler molecules. The part of the enzyme that binds the substrate to be acted upon is referred to as the active site. ...
Clinical biochemistry (9) Enzymes and isoenzymes
... that combine with the enzyme and prevent normal ES interactions in the active site, thus diminishing the rate of the reaction. 1) Competitive inhibition. 2) Non-competitive inhibition. 3) Mixed inhibition. ...
... that combine with the enzyme and prevent normal ES interactions in the active site, thus diminishing the rate of the reaction. 1) Competitive inhibition. 2) Non-competitive inhibition. 3) Mixed inhibition. ...
Enzymes
... diffused into the osmometer and WHY? This is a two part question. 5. Molecules diffuse from an area of [high] [low] OR [low] [high]? (note [ ] = concentration) ...
... diffused into the osmometer and WHY? This is a two part question. 5. Molecules diffuse from an area of [high] [low] OR [low] [high]? (note [ ] = concentration) ...
Enzyme kinetics

Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or an agonist might inhibit the enzyme.Enzymes are usually protein molecules that manipulate other molecules — the enzymes' substrates. These target molecules bind to an enzyme's active site and are transformed into products through a series of steps known as the enzymatic mechanismE + S <——> ES <——> ES*< ——> EP <——> E + P. These mechanisms can be divided into single-substrate and multiple-substrate mechanisms. Kinetic studies on enzymes that only bind one substrate, such as triosephosphate isomerase, aim to measure the affinity with which the enzyme binds this substrate and the turnover rate. Some other examples of enzymes are phosphofructokinase and hexokinase, both of which are important for cellular respiration (glycolysis).When enzymes bind multiple substrates, such as dihydrofolate reductase (shown right), enzyme kinetics can also show the sequence in which these substrates bind and the sequence in which products are released. An example of enzymes that bind a single substrate and release multiple products are proteases, which cleave one protein substrate into two polypeptide products. Others join two substrates together, such as DNA polymerase linking a nucleotide to DNA. Although these mechanisms are often a complex series of steps, there is typically one rate-determining step that determines the overall kinetics. This rate-determining step may be a chemical reaction or a conformational change of the enzyme or substrates, such as those involved in the release of product(s) from the enzyme.Knowledge of the enzyme's structure is helpful in interpreting kinetic data. For example, the structure can suggest how substrates and products bind during catalysis; what changes occur during the reaction; and even the role of particular amino acid residues in the mechanism. Some enzymes change shape significantly during the mechanism; in such cases, it is helpful to determine the enzyme structure with and without bound substrate analogues that do not undergo the enzymatic reaction.Not all biological catalysts are protein enzymes; RNA-based catalysts such as ribozymes and ribosomes are essential to many cellular functions, such as RNA splicing and translation. The main difference between ribozymes and enzymes is that RNA catalysts are composed of nucleotides, whereas enzymes are composed of amino acids. Ribozymes also perform a more limited set of reactions, although their reaction mechanisms and kinetics can be analysed and classified by the same methods.