Enzymes are NOT reactants!
... [Enzyme inhibitors]: change shape of active site and shut off activity a) [Competitive inhibitors]: Competitor molecule binds at active site, prevents substrate from binding. b) [Non-competitive inhibitors]: End product binds at a allosteric (different site), changes shape of active site. Substrate ...
... [Enzyme inhibitors]: change shape of active site and shut off activity a) [Competitive inhibitors]: Competitor molecule binds at active site, prevents substrate from binding. b) [Non-competitive inhibitors]: End product binds at a allosteric (different site), changes shape of active site. Substrate ...
Enzyme Catalysis
... within cells is to prevent the accumulation of toxic levels of hydrogen peroxide formed as a byproduct of metabolic processes. Catalase might also take part in some of the many oxidation reactions that occur in the cell. 2H2O2 -------> 2 H2O + O2 (gas ) In the absence of catalase, this reaction occu ...
... within cells is to prevent the accumulation of toxic levels of hydrogen peroxide formed as a byproduct of metabolic processes. Catalase might also take part in some of the many oxidation reactions that occur in the cell. 2H2O2 -------> 2 H2O + O2 (gas ) In the absence of catalase, this reaction occu ...
Chapter 5: Enzymes
... 3. Oxidation of Glucose Release of Energy to do work Involves a series of enzyme catalysed reactions 4. Breakdown of toxic materials Hydrogen peroxide to water and oxygen catalysed by catalase. Enzymes catalyse almost all reactions in body. There are many different types of enzymes and each is ...
... 3. Oxidation of Glucose Release of Energy to do work Involves a series of enzyme catalysed reactions 4. Breakdown of toxic materials Hydrogen peroxide to water and oxygen catalysed by catalase. Enzymes catalyse almost all reactions in body. There are many different types of enzymes and each is ...
Enzymes
... 1. Why can pepsin(an enzyme) digest proteins not carbohydrate or lipid in stomach? How can pepsin be active in stomach but not in mouth or small intestine? An enzyme including pepsin reacts only with specific substrates. Stomach is acidic, the pH which pepsin has optimum activity. 2. Match the term. ...
... 1. Why can pepsin(an enzyme) digest proteins not carbohydrate or lipid in stomach? How can pepsin be active in stomach but not in mouth or small intestine? An enzyme including pepsin reacts only with specific substrates. Stomach is acidic, the pH which pepsin has optimum activity. 2. Match the term. ...
3 - IBperiod5
... 3.6.3 Explain the effects of temperature, pH and substrate concentration on enzyme activity. [Enzyme activity could be measured using data loggers such as pressure sensors, pH sensors, or coloromiters. The effects of environmental acid rain could be discussed.] Temperature and pH can change the shap ...
... 3.6.3 Explain the effects of temperature, pH and substrate concentration on enzyme activity. [Enzyme activity could be measured using data loggers such as pressure sensors, pH sensors, or coloromiters. The effects of environmental acid rain could be discussed.] Temperature and pH can change the shap ...
J. Anim. Sci. Vol. 80, Suppl. 1/J. Dairy Sci. Vol. 85, Suppl. 1 414 Use
... the diet. When viewed across a variety of enzyme products and experimental conditions the response to feed enzymes by ruminants has been variable. This variation can be attributed to experimental conditions in which energy is not the limiting nutrient, as well as the activities and characteristics o ...
... the diet. When viewed across a variety of enzyme products and experimental conditions the response to feed enzymes by ruminants has been variable. This variation can be attributed to experimental conditions in which energy is not the limiting nutrient, as well as the activities and characteristics o ...
BIOCHEMISTRY (CHEM 360)
... tightly bound the enzyme substrate complex is. The enzyme substrate complex is tightly bound if the relative magnitude(s) of the following rate constant(s) is/are high “ k1” (fill in the blanks) The enzyme substrate complex is loosely bound if the relative magnitude(s) of the following rate constant ...
... tightly bound the enzyme substrate complex is. The enzyme substrate complex is tightly bound if the relative magnitude(s) of the following rate constant(s) is/are high “ k1” (fill in the blanks) The enzyme substrate complex is loosely bound if the relative magnitude(s) of the following rate constant ...
Enzyme Lab Period _____ Date
... molecules. Enzymes are extremely efficient and may be used over and over again. One enzyme may catalyze thousands of reactions every second. Both the temperature and the pH at which enzymes function are extremely important. Most organisms have a preferred temperature range in which they survive, and ...
... molecules. Enzymes are extremely efficient and may be used over and over again. One enzyme may catalyze thousands of reactions every second. Both the temperature and the pH at which enzymes function are extremely important. Most organisms have a preferred temperature range in which they survive, and ...
Diagnosis Test: EDEXCEL ADDITIONAL SCIENCE Biology
... 2. Complete the sentences with the words in the box below; the words may be used once, more than once or not at all. ...
... 2. Complete the sentences with the words in the box below; the words may be used once, more than once or not at all. ...
Biological Oscillations
... • Three equations are needed. Both DNA and substrate concentrations are assumed to be at much greater concentrations than mRNA, the enzyme machinery and the product made. ...
... • Three equations are needed. Both DNA and substrate concentrations are assumed to be at much greater concentrations than mRNA, the enzyme machinery and the product made. ...
Event Poster PDF
... be explained by the need for an open site to bind substrate, but a closed state to align residues for catalysis. Enzyme specificity is a kinetic phenomena that cannot be addressed by measurements at equilibrium. Fersht argued that a two-step substrate binding reaction involving a change in enzyme st ...
... be explained by the need for an open site to bind substrate, but a closed state to align residues for catalysis. Enzyme specificity is a kinetic phenomena that cannot be addressed by measurements at equilibrium. Fersht argued that a two-step substrate binding reaction involving a change in enzyme st ...
Enzymes - Deans Community High School
... What does the term metabolism mean? Draw a diagram showing the biochemical pathway in which compound P is converted to compound Q by the action of enzyme Y. Compound Q can then be converted to compound R by the action of enzyme Z. 5. What happens within the cells of someone that does not produce enz ...
... What does the term metabolism mean? Draw a diagram showing the biochemical pathway in which compound P is converted to compound Q by the action of enzyme Y. Compound Q can then be converted to compound R by the action of enzyme Z. 5. What happens within the cells of someone that does not produce enz ...
Factors affecting Enzyme Activity
... The activity of an Enzyme is affected by its environmental conditions. Changing these alter the rate of reaction caused by the enzyme. In nature, organisms adjust the conditions of their enzymes to produce anOptimum rate of reaction, where necessary, or they may have enzymes which are adapted to fun ...
... The activity of an Enzyme is affected by its environmental conditions. Changing these alter the rate of reaction caused by the enzyme. In nature, organisms adjust the conditions of their enzymes to produce anOptimum rate of reaction, where necessary, or they may have enzymes which are adapted to fun ...
Enzyme Activity
... • Active site: The region of an enzyme molecule which binds the substrate and carries out the catalytic reaction • Enzyme : A biological catalyst. Usually a globular protein molecule produced by living organisms that can speed up a specific chemical reaction without itself being destroyed or changed ...
... • Active site: The region of an enzyme molecule which binds the substrate and carries out the catalytic reaction • Enzyme : A biological catalyst. Usually a globular protein molecule produced by living organisms that can speed up a specific chemical reaction without itself being destroyed or changed ...
enzymes - SD57 Mail
... genetically controlled (control of protein synthesis) • Increasing the amount of enzyme will increase the reaction rate (as long as substrate is present) ...
... genetically controlled (control of protein synthesis) • Increasing the amount of enzyme will increase the reaction rate (as long as substrate is present) ...
Questions 1-5: True or False? (2 points each) 1. The reaction
... Questions 11-16: True or False? (2 points each) 11. In the SN1 mechanism proposed for lysozyme, the enzyme forms a covalent bond with the substrate. 12. The reaction catalyzed by lysozyme can be considered a group transfer reaction. 13. Lysozyme is a glycosidase. 14. Chymotrypsin catalyzes a ping-po ...
... Questions 11-16: True or False? (2 points each) 11. In the SN1 mechanism proposed for lysozyme, the enzyme forms a covalent bond with the substrate. 12. The reaction catalyzed by lysozyme can be considered a group transfer reaction. 13. Lysozyme is a glycosidase. 14. Chymotrypsin catalyzes a ping-po ...
Questions for Enzyme - I
... 8. Following statement is true regarding Km Value a. Signifies the affinity of enzyme for substrate b. Never altered by enzyme inhibitors c. It is the conc. Of enzyme at half maximal velocity d. One enzyme can have different Km values with same substrate ...
... 8. Following statement is true regarding Km Value a. Signifies the affinity of enzyme for substrate b. Never altered by enzyme inhibitors c. It is the conc. Of enzyme at half maximal velocity d. One enzyme can have different Km values with same substrate ...
Enzymes and Activation Energy
... • Notice the optimum (best) activity level for each enzyme matches the environment that the enzyme has to work in. • Human enzymes generally work best at our temperature (37°C) while the thermophilic bacteria's enzyme works best at a higher temperature. • Indeed some of these thermophilic organisms ...
... • Notice the optimum (best) activity level for each enzyme matches the environment that the enzyme has to work in. • Human enzymes generally work best at our temperature (37°C) while the thermophilic bacteria's enzyme works best at a higher temperature. • Indeed some of these thermophilic organisms ...
enzymes - iLearning Centre
... Increase in substrate concentration, more substrate molecule are available to bind the active sites of the enzyme Hence, more products will be produced Because more chances of collision between the substrate molecule and the enzyme molecules for a catalytic reaction to take place In increase in subs ...
... Increase in substrate concentration, more substrate molecule are available to bind the active sites of the enzyme Hence, more products will be produced Because more chances of collision between the substrate molecule and the enzyme molecules for a catalytic reaction to take place In increase in subs ...
molecular adaptation to temperature
... 1. Higher temperatures require increased thermal stability of proteins (i.e., enzymes) and this increased stability hinders conformational changes that occur during catalysis. 2. More weak bonds between enzyme and substrate must be formed at higher temperatures to hold the ligand (substrates) to the ...
... 1. Higher temperatures require increased thermal stability of proteins (i.e., enzymes) and this increased stability hinders conformational changes that occur during catalysis. 2. More weak bonds between enzyme and substrate must be formed at higher temperatures to hold the ligand (substrates) to the ...
Enzyme Class
... which functions as a biological catalyst, speeding up reaction rate by lowering activation energy without being affected by the reaction it catalyse ...
... which functions as a biological catalyst, speeding up reaction rate by lowering activation energy without being affected by the reaction it catalyse ...
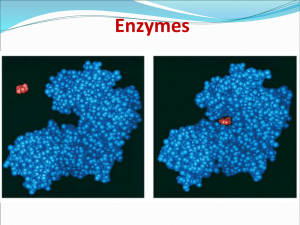
Enzymes
... • Enzymes are not changed by the reaction – used only temporarily – re-used again for the same reaction with other molecules – very little enzyme needed to help in many reactions substrate active site ...
... • Enzymes are not changed by the reaction – used only temporarily – re-used again for the same reaction with other molecules – very little enzyme needed to help in many reactions substrate active site ...
Enzymes
... • Enzymes are not changed by the reaction – used only temporarily – re-used again for the same reaction with other molecules – very little enzyme needed to help in many reactions substrate active site ...
... • Enzymes are not changed by the reaction – used only temporarily – re-used again for the same reaction with other molecules – very little enzyme needed to help in many reactions substrate active site ...
Enzymes - Chautauqua Lake Central SD
... • Enzymes are not changed by the reaction – used only temporarily – re-used again for the same reaction with other molecules – very little enzyme needed to help in many reactions substrate active site ...
... • Enzymes are not changed by the reaction – used only temporarily – re-used again for the same reaction with other molecules – very little enzyme needed to help in many reactions substrate active site ...
Enzyme kinetics

Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or an agonist might inhibit the enzyme.Enzymes are usually protein molecules that manipulate other molecules — the enzymes' substrates. These target molecules bind to an enzyme's active site and are transformed into products through a series of steps known as the enzymatic mechanismE + S <——> ES <——> ES*< ——> EP <——> E + P. These mechanisms can be divided into single-substrate and multiple-substrate mechanisms. Kinetic studies on enzymes that only bind one substrate, such as triosephosphate isomerase, aim to measure the affinity with which the enzyme binds this substrate and the turnover rate. Some other examples of enzymes are phosphofructokinase and hexokinase, both of which are important for cellular respiration (glycolysis).When enzymes bind multiple substrates, such as dihydrofolate reductase (shown right), enzyme kinetics can also show the sequence in which these substrates bind and the sequence in which products are released. An example of enzymes that bind a single substrate and release multiple products are proteases, which cleave one protein substrate into two polypeptide products. Others join two substrates together, such as DNA polymerase linking a nucleotide to DNA. Although these mechanisms are often a complex series of steps, there is typically one rate-determining step that determines the overall kinetics. This rate-determining step may be a chemical reaction or a conformational change of the enzyme or substrates, such as those involved in the release of product(s) from the enzyme.Knowledge of the enzyme's structure is helpful in interpreting kinetic data. For example, the structure can suggest how substrates and products bind during catalysis; what changes occur during the reaction; and even the role of particular amino acid residues in the mechanism. Some enzymes change shape significantly during the mechanism; in such cases, it is helpful to determine the enzyme structure with and without bound substrate analogues that do not undergo the enzymatic reaction.Not all biological catalysts are protein enzymes; RNA-based catalysts such as ribozymes and ribosomes are essential to many cellular functions, such as RNA splicing and translation. The main difference between ribozymes and enzymes is that RNA catalysts are composed of nucleotides, whereas enzymes are composed of amino acids. Ribozymes also perform a more limited set of reactions, although their reaction mechanisms and kinetics can be analysed and classified by the same methods.