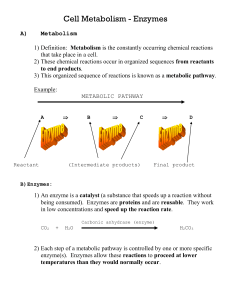
Enzymes
... Also spectroscopic characteristics of enzymes and/or substrates sometimes change upon the formation of the ES complex. It is particularly obvious if there is a colored prosthetic group bound to an enzyme. Trp synthetase uses a pyridoxal phosphate (PLP) prosthetic group to make L-Trp from Ser and in ...
... Also spectroscopic characteristics of enzymes and/or substrates sometimes change upon the formation of the ES complex. It is particularly obvious if there is a colored prosthetic group bound to an enzyme. Trp synthetase uses a pyridoxal phosphate (PLP) prosthetic group to make L-Trp from Ser and in ...
Enzymes - Kevan Kruger
... 2) Usually, heat is used to speed up chemical reactions by increasing the number of collisions that occur between reactants. Excessive heat, however, destroys the tertiary structure of protein (denatures) and therefore cannot be used to speed up reactions within living organisms. 3) Enzymes act as ...
... 2) Usually, heat is used to speed up chemical reactions by increasing the number of collisions that occur between reactants. Excessive heat, however, destroys the tertiary structure of protein (denatures) and therefore cannot be used to speed up reactions within living organisms. 3) Enzymes act as ...
Essential Questions: What is an enzyme? How do enzymes work
... Wait, What is a Chemical Reaction? • The process of changing one set of chemicals (reactants) into another set of chemicals (products) by rearranging the atoms. • The bonds joining the reactants are broken and new bonds are formed in the products. • All life processes are driven by chemical ...
... Wait, What is a Chemical Reaction? • The process of changing one set of chemicals (reactants) into another set of chemicals (products) by rearranging the atoms. • The bonds joining the reactants are broken and new bonds are formed in the products. • All life processes are driven by chemical ...
Enzymes! - Mrs. Ahrens` Science Site
... site which becomes the enzymesubstrate complex. • The shape of an enzyme is so specific that generally only one enzyme will work for one substrate(s). ...
... site which becomes the enzymesubstrate complex. • The shape of an enzyme is so specific that generally only one enzyme will work for one substrate(s). ...
Chapter 3-5 Organic Chemistry
... Partly a function of the initial concentration of a substrate • More substrate = more frequently access active sites of enzyme • There is a limit to this… • Sometimes all enzymes are “busy” • Enzyme is said to be “saturated” ...
... Partly a function of the initial concentration of a substrate • More substrate = more frequently access active sites of enzyme • There is a limit to this… • Sometimes all enzymes are “busy” • Enzyme is said to be “saturated” ...
enzymes - onlinebiosurgery
... How do enzymes work? • The molecules that react in the enzyme-catalysed reaction are substrates • Molecules produced in the reaction are products • The active site is the part of the enzyme where the substrate fits and product forms • Just like a key only fits into a specific lock, each enzyme has ...
... How do enzymes work? • The molecules that react in the enzyme-catalysed reaction are substrates • Molecules produced in the reaction are products • The active site is the part of the enzyme where the substrate fits and product forms • Just like a key only fits into a specific lock, each enzyme has ...
The Kinetics of Enzyme Catalyzed Reactions
... small portion of the surface of an enzyme which a specific chemical reaction is catalyzed • substrate - the molecule being utilized and/or modified by a particular enzyme at its active site • co-factor - organic or inorganic molecules that are required by some enzymes for activity. These include Mg2 ...
... small portion of the surface of an enzyme which a specific chemical reaction is catalyzed • substrate - the molecule being utilized and/or modified by a particular enzyme at its active site • co-factor - organic or inorganic molecules that are required by some enzymes for activity. These include Mg2 ...
Catalysts in biochemical reactions
... (in a random mechanism) or substrates have to bind in a particular sequence (in an ordered mechanism). When a set of v by [S] curves (fixed A, varying B) from an enzyme with a ternary-complex mechanism are plotted in a Line weaver-Burk plot the set of lines produced will intersect. Enzymes with tern ...
... (in a random mechanism) or substrates have to bind in a particular sequence (in an ordered mechanism). When a set of v by [S] curves (fixed A, varying B) from an enzyme with a ternary-complex mechanism are plotted in a Line weaver-Burk plot the set of lines produced will intersect. Enzymes with tern ...
1.3 Enzymes supplemental work
... can have on biological processes and how environments can have an effect on the activity of enzymes ...
... can have on biological processes and how environments can have an effect on the activity of enzymes ...
Enzymes: Introduction • Enzymes are catalysts which speed up the
... Properties of enzyme: Enzymes work at optimal p H and temperature making them most environmentally friendly solution for industrial manufacturing. Enzymes are biodegradable and keep on working until they are dissolved usually by other enzymes. All enzymes are proteins but not all proteins ar ...
... Properties of enzyme: Enzymes work at optimal p H and temperature making them most environmentally friendly solution for industrial manufacturing. Enzymes are biodegradable and keep on working until they are dissolved usually by other enzymes. All enzymes are proteins but not all proteins ar ...
Simple kinetics of enzyme action
... even wider range of enzyme concentrations than allowed in closed systems, and is commonly used to model immobilised enzyme kinetic systems (see Chapter 3). Enzymes have evolved by maximising kcat/K m (i.e. the specificity constant for the substrate) whilst keeping K m approximately identical to the ...
... even wider range of enzyme concentrations than allowed in closed systems, and is commonly used to model immobilised enzyme kinetic systems (see Chapter 3). Enzymes have evolved by maximising kcat/K m (i.e. the specificity constant for the substrate) whilst keeping K m approximately identical to the ...
Biochemistry and Enzymes - St. John the Baptist Diocesan High
... Enzymes work by lowering the activation energy of chemical reactions. This means it lowers the amount of energy needed to start a chemical reaction, so it occurs faster! ...
... Enzymes work by lowering the activation energy of chemical reactions. This means it lowers the amount of energy needed to start a chemical reaction, so it occurs faster! ...
Catalase Lab How do enzymes work in living tissues? Introduction
... What would happen to your cells if they made a poisonous chemical? You might think that they would die. In fact, your cells are always making poisonous chemical. They do not die because your cells use enzymes to break down these poisonous chemicals into harmless substances. Enzymes are proteins that ...
... What would happen to your cells if they made a poisonous chemical? You might think that they would die. In fact, your cells are always making poisonous chemical. They do not die because your cells use enzymes to break down these poisonous chemicals into harmless substances. Enzymes are proteins that ...
Lecture 2 * The Kinetics of Enzyme Catalyzed
... • The enzyme activity does not increase continuously as the temperature is raised. Instead, the enzyme usually loses activity at quite a low temperature, often only slightly above that at which it is typically found. ...
... • The enzyme activity does not increase continuously as the temperature is raised. Instead, the enzyme usually loses activity at quite a low temperature, often only slightly above that at which it is typically found. ...
Exercise 7. Enzyme Kinetics
... 2) In the procedure text, the Michaelis Menten equation is derived assuming a rapid equilibrium step. Show the derivation for an alternative form, instead assuming a pseudosteady state. Experimentally, how could one test verify the accuracy of this assumption if direct observation of the intermediat ...
... 2) In the procedure text, the Michaelis Menten equation is derived assuming a rapid equilibrium step. Show the derivation for an alternative form, instead assuming a pseudosteady state. Experimentally, how could one test verify the accuracy of this assumption if direct observation of the intermediat ...
ANSWERS TO REVIEW QUESTIONS – CHAPTER 03
... What is the basis of the specificity of enzyme action? Explain in terms of enzyme structure why boiling inactivates enzymes. (pp. 60–65) An enzyme is a catalyst produced by the protein production machinery of a cell. It acts by increasing the rate of particular chemical reactions. The basis for enzy ...
... What is the basis of the specificity of enzyme action? Explain in terms of enzyme structure why boiling inactivates enzymes. (pp. 60–65) An enzyme is a catalyst produced by the protein production machinery of a cell. It acts by increasing the rate of particular chemical reactions. The basis for enzy ...
Effect aliphatic alcohols on catalytic activity of bovine pancreatic α
... organic media provide the possibility of conducting industrially important synthetic reactions that do not occur in aqueous media (peptide synthesis and esterification). The enzymatic catalysis in organic solvents is competitive and cost-saving technology for producing substances with a high optical ...
... organic media provide the possibility of conducting industrially important synthetic reactions that do not occur in aqueous media (peptide synthesis and esterification). The enzymatic catalysis in organic solvents is competitive and cost-saving technology for producing substances with a high optical ...
Dioxygen Binding in the Active Site of Histone Demethylase
... Now we have studied the first step of demethylation by JmjC proteins, highlighting the importance of non-local energy contributions from residues and substrate. Our next steps will involve understanding the structural basis for substrate selectivity within the active site, and the full reaction coor ...
... Now we have studied the first step of demethylation by JmjC proteins, highlighting the importance of non-local energy contributions from residues and substrate. Our next steps will involve understanding the structural basis for substrate selectivity within the active site, and the full reaction coor ...
File - need help with revision notes?
... act as protease inhibitors. These prevent the viruses from replicating by inhibiting the activity of the protease enzyme, which the viruses need in order to build new virus coats. o Antibiotics as a competitive inhibitor: Penicillin Penicillin is an inhibitor of a bacterial enzyme that forms cross ...
... act as protease inhibitors. These prevent the viruses from replicating by inhibiting the activity of the protease enzyme, which the viruses need in order to build new virus coats. o Antibiotics as a competitive inhibitor: Penicillin Penicillin is an inhibitor of a bacterial enzyme that forms cross ...
Bio 114: Virtual Enzyme Lab
... http://bioweb.wku.edu/courses/Biol120/Web/enzyme1b.asp Use Internet Explorer 8. Why is it important to let the test tubes “acclimate” to the temperature of the ...
... http://bioweb.wku.edu/courses/Biol120/Web/enzyme1b.asp Use Internet Explorer 8. Why is it important to let the test tubes “acclimate” to the temperature of the ...
enzyme - iGEM 2014
... substrate occurs in the transition-state (ES‡) • The enzyme distorts the substrate, forcing it toward the transition state • An enzyme must be complementary to the transition-state in shape and chemical character • Enzymes may bind their transition states 1010 to 1015 times more tightly than their s ...
... substrate occurs in the transition-state (ES‡) • The enzyme distorts the substrate, forcing it toward the transition state • An enzyme must be complementary to the transition-state in shape and chemical character • Enzymes may bind their transition states 1010 to 1015 times more tightly than their s ...
Enzymes Notes
... ENZYMES' ! Enzymes'are'PROTEINS"' ! Enzymes'are'biological' CATALYSTS'that'speed%up% chemical%reac.ons'and' decrease/lower'the' acAvaAon'energy'while' releasing'energy.' ! Enzymes'affect'the'reacAons'in' living'cells'by'changing'the' speed'of'the'reacAon' ...
... ENZYMES' ! Enzymes'are'PROTEINS"' ! Enzymes'are'biological' CATALYSTS'that'speed%up% chemical%reac.ons'and' decrease/lower'the' acAvaAon'energy'while' releasing'energy.' ! Enzymes'affect'the'reacAons'in' living'cells'by'changing'the' speed'of'the'reacAon' ...
Enzyme kinetics

Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or an agonist might inhibit the enzyme.Enzymes are usually protein molecules that manipulate other molecules — the enzymes' substrates. These target molecules bind to an enzyme's active site and are transformed into products through a series of steps known as the enzymatic mechanismE + S <——> ES <——> ES*< ——> EP <——> E + P. These mechanisms can be divided into single-substrate and multiple-substrate mechanisms. Kinetic studies on enzymes that only bind one substrate, such as triosephosphate isomerase, aim to measure the affinity with which the enzyme binds this substrate and the turnover rate. Some other examples of enzymes are phosphofructokinase and hexokinase, both of which are important for cellular respiration (glycolysis).When enzymes bind multiple substrates, such as dihydrofolate reductase (shown right), enzyme kinetics can also show the sequence in which these substrates bind and the sequence in which products are released. An example of enzymes that bind a single substrate and release multiple products are proteases, which cleave one protein substrate into two polypeptide products. Others join two substrates together, such as DNA polymerase linking a nucleotide to DNA. Although these mechanisms are often a complex series of steps, there is typically one rate-determining step that determines the overall kinetics. This rate-determining step may be a chemical reaction or a conformational change of the enzyme or substrates, such as those involved in the release of product(s) from the enzyme.Knowledge of the enzyme's structure is helpful in interpreting kinetic data. For example, the structure can suggest how substrates and products bind during catalysis; what changes occur during the reaction; and even the role of particular amino acid residues in the mechanism. Some enzymes change shape significantly during the mechanism; in such cases, it is helpful to determine the enzyme structure with and without bound substrate analogues that do not undergo the enzymatic reaction.Not all biological catalysts are protein enzymes; RNA-based catalysts such as ribozymes and ribosomes are essential to many cellular functions, such as RNA splicing and translation. The main difference between ribozymes and enzymes is that RNA catalysts are composed of nucleotides, whereas enzymes are composed of amino acids. Ribozymes also perform a more limited set of reactions, although their reaction mechanisms and kinetics can be analysed and classified by the same methods.