Example4
... CHALLENGE ACTIVITY Group 1 Challenge! Which organelles involved in the synthesis of pepsin; a digestive enzyme formed by stomach cells. ...
... CHALLENGE ACTIVITY Group 1 Challenge! Which organelles involved in the synthesis of pepsin; a digestive enzyme formed by stomach cells. ...
Document
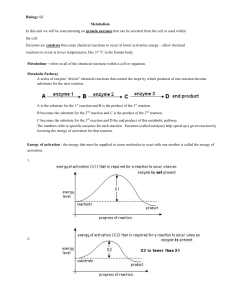
... The last of these assumptions(Eq. 4) is not fulfilled in the initial and final stage of the reaction. However this initial phase is so fast, that is not measurable by means of traditional methods (with manual mixing of reagents in experimental cuvette). Studying of this initial, rapid stage of react ...
... The last of these assumptions(Eq. 4) is not fulfilled in the initial and final stage of the reaction. However this initial phase is so fast, that is not measurable by means of traditional methods (with manual mixing of reagents in experimental cuvette). Studying of this initial, rapid stage of react ...
• Warm-up What are the four macromolecules and their function?
... • A few enzymes (such as gastric protease) work best at a pH of about 2.0 ...
... • A few enzymes (such as gastric protease) work best at a pH of about 2.0 ...
Organic Molecules
... Substrate – the “reactants” . These bind to the enzyme Active Site – where the “reactants” bind to the enzyme Products – what is formed during the reaction There are many different enzymes, but each one will only fit one substrate. Like a lock and key. ...
... Substrate – the “reactants” . These bind to the enzyme Active Site – where the “reactants” bind to the enzyme Products – what is formed during the reaction There are many different enzymes, but each one will only fit one substrate. Like a lock and key. ...
Camp 1
... • Lock-and-key model & Induced-fit model emphasize the shape of the active site. • However, Chemistry of active site is most important. • Just 5 amino acids participate in active sites of: ≤65% of the enzymes studies to date. ...
... • Lock-and-key model & Induced-fit model emphasize the shape of the active site. • However, Chemistry of active site is most important. • Just 5 amino acids participate in active sites of: ≤65% of the enzymes studies to date. ...
Enzyme Mechanisms
... system is the ribosome. The central catalytic activity of the ribosome (peptide bond formation) is catalyzed by an RNA component. ...
... system is the ribosome. The central catalytic activity of the ribosome (peptide bond formation) is catalyzed by an RNA component. ...
Enzyme Notes Outline
... When are they used?: To speed up chemical reactions are too slow or have high activation energies ** Enzymes are very specific- they only catalyze one type of reaction ** What is the “substrate”-The reactants that interact with enzymes What is the “active site”-Site of the chemical reaction. Explain ...
... When are they used?: To speed up chemical reactions are too slow or have high activation energies ** Enzymes are very specific- they only catalyze one type of reaction ** What is the “substrate”-The reactants that interact with enzymes What is the “active site”-Site of the chemical reaction. Explain ...
CLS 1113 Introduction to Clinical Laboratory Practices
... Readily available Long shelf life Easily adapted to automation ...
... Readily available Long shelf life Easily adapted to automation ...
Biology - WordPress.com
... Speed up rate of reaction and lower activation energy 2. Where does a substrate attach to an enzyme?_________________ Active site Activation energy 3. If an enzyme is present in a reaction, less ______________________ is needed to get the reaction started. ...
... Speed up rate of reaction and lower activation energy 2. Where does a substrate attach to an enzyme?_________________ Active site Activation energy 3. If an enzyme is present in a reaction, less ______________________ is needed to get the reaction started. ...
AP Bio – Enzyme Activity This activity is an alternative to the
... 0 units / mL 2. Prepare 40 mL of enzyme for each of the above concentrations in the following manner: The stock solution for the class is 100%. Distilled water is 0%. Add equal amounts of catalase and water and you have 50%. Add equal amounts of the 50% and 100% and you have 75%. Likewise, mix equal ...
... 0 units / mL 2. Prepare 40 mL of enzyme for each of the above concentrations in the following manner: The stock solution for the class is 100%. Distilled water is 0%. Add equal amounts of catalase and water and you have 50%. Add equal amounts of the 50% and 100% and you have 75%. Likewise, mix equal ...
File
... Reactions without an enzyme: require more energy to start Reactions with an enzyme: require less energy to start ...
... Reactions without an enzyme: require more energy to start Reactions with an enzyme: require less energy to start ...
Lecture 11 Enzymes: Kinetics
... • kcat/Km can never be greater than k1 (rate constant for association of E + S) for a given enzyme-substrate system. (You're not responsible for the explanation of this.) – How fast 2 molecules can react (or bind in the case of E + S) is limited by how fast the molecules can diffuse to "bump into ea ...
... • kcat/Km can never be greater than k1 (rate constant for association of E + S) for a given enzyme-substrate system. (You're not responsible for the explanation of this.) – How fast 2 molecules can react (or bind in the case of E + S) is limited by how fast the molecules can diffuse to "bump into ea ...
Both PS 7 and PS 8 are due next Thursday
... KM = 1.8 x 10-5 M When [glyceraldehyde-3-P] = 30 µM, v = 82.5 µmol mL-1 sec-1 What is Vmax for the triose phosphate isomerase? Assume [ET] = 3 nmol/mL, what is kcat for triose phosphate ...
... KM = 1.8 x 10-5 M When [glyceraldehyde-3-P] = 30 µM, v = 82.5 µmol mL-1 sec-1 What is Vmax for the triose phosphate isomerase? Assume [ET] = 3 nmol/mL, what is kcat for triose phosphate ...
Factors Affecting the Rate of Enzyme Reaction in Liver
... increases the rate of chemical reaction by lowering the level of activation energy necessary to start the reaction. Without enzymes, many of the chemical reactions that occur within living things would proceed too slowly to be useful. Enzymes speed up these reactions by bringing the reactants into c ...
... increases the rate of chemical reaction by lowering the level of activation energy necessary to start the reaction. Without enzymes, many of the chemical reactions that occur within living things would proceed too slowly to be useful. Enzymes speed up these reactions by bringing the reactants into c ...
Enzyme Kinetics
... state. The kinetic mechanism derived from steady state kinetics allows one to specify the order of addition of substrates and order of release of products. However, steps in the reaction are not defined or directly measured. Direct measurements of reactions at the enzyme active site are necessary to ...
... state. The kinetic mechanism derived from steady state kinetics allows one to specify the order of addition of substrates and order of release of products. However, steps in the reaction are not defined or directly measured. Direct measurements of reactions at the enzyme active site are necessary to ...
can
... Two different therapeutic approaches for treating: A. A DNA virus (e.g. Herpes) B. An RNA virus (e.g. Influenza) ...
... Two different therapeutic approaches for treating: A. A DNA virus (e.g. Herpes) B. An RNA virus (e.g. Influenza) ...
Adding Enzymes To Dairy Diets
... conditions differs widely among enzymes. Commercial enzyme products marketed as feed supplements are bacterial or fungal fermentation extracts. These preparations vary widely in the types and concentrations of specific enzymes they contain. As a result, their ability to digest particular feeds is al ...
... conditions differs widely among enzymes. Commercial enzyme products marketed as feed supplements are bacterial or fungal fermentation extracts. These preparations vary widely in the types and concentrations of specific enzymes they contain. As a result, their ability to digest particular feeds is al ...
f212 molecules biodiversity food health 2.1.3 enzymes
... its volume can be measured. • It’s also common to investigate the effect that changing the enzyme or substrate concentration has on the rate of this reaction. ...
... its volume can be measured. • It’s also common to investigate the effect that changing the enzyme or substrate concentration has on the rate of this reaction. ...
Metabolism, Energy and Enzymes Team – Game – Tournament
... 38. Why are product molecules released from the enzyme’s active site? 39. What are the non-protein, organic molecules that assist enzymes in catalyzing a biochemical reaction? 40. What are the metal, non-organic molecules that assist enzymes in catalyzing a biochemical reaction? 41. State one of the ...
... 38. Why are product molecules released from the enzyme’s active site? 39. What are the non-protein, organic molecules that assist enzymes in catalyzing a biochemical reaction? 40. What are the metal, non-organic molecules that assist enzymes in catalyzing a biochemical reaction? 41. State one of the ...
ENZYME ACTIVITY INQUIRY LAB
... For example, if potato cells are crushed or cut into smaller pieces, this may affect how much catalase enzyme comes into contact with substrate (H2O2). ...
... For example, if potato cells are crushed or cut into smaller pieces, this may affect how much catalase enzyme comes into contact with substrate (H2O2). ...
enzyme names end in “ase”
... LD1 was first discovered in the heart and has four (4) identical protein sub-units. Since they are identical and from the heart, each sub-unit is called an “H” sub-unit. There are four (4) H subunits in LD1. LD5 was identified in muscle and it, too, had four (4) identical sub-units, called “M” for m ...
... LD1 was first discovered in the heart and has four (4) identical protein sub-units. Since they are identical and from the heart, each sub-unit is called an “H” sub-unit. There are four (4) H subunits in LD1. LD5 was identified in muscle and it, too, had four (4) identical sub-units, called “M” for m ...
Enzymes & pH - SchoolWorld an Edline Solution
... http://lpscience.fatcow.com/jwanamaker/animations/Enzyme%20activity.html ...
... http://lpscience.fatcow.com/jwanamaker/animations/Enzyme%20activity.html ...
Slide 1
... Without enzymes, more energy would be required in order for reactants molecules to collide with one another. When a chemical reaction involves two or more reactants, the enzyme provides a site where the reactants are positioned very close to each other and in an orientation that facilitates the form ...
... Without enzymes, more energy would be required in order for reactants molecules to collide with one another. When a chemical reaction involves two or more reactants, the enzyme provides a site where the reactants are positioned very close to each other and in an orientation that facilitates the form ...
Course Notes
... 2 wastes products (H2O + CO2) 2. If O2 is not available then glucose will be metabolized to lactic acid. This reaction produces a maximum of 4 ATP and is called anaerobic cellular respiration. The waste product lactic acid causes pH to drop in the surrounding tissue and blood. The point at which lac ...
... 2 wastes products (H2O + CO2) 2. If O2 is not available then glucose will be metabolized to lactic acid. This reaction produces a maximum of 4 ATP and is called anaerobic cellular respiration. The waste product lactic acid causes pH to drop in the surrounding tissue and blood. The point at which lac ...
Enzyme kinetics

Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or an agonist might inhibit the enzyme.Enzymes are usually protein molecules that manipulate other molecules — the enzymes' substrates. These target molecules bind to an enzyme's active site and are transformed into products through a series of steps known as the enzymatic mechanismE + S <——> ES <——> ES*< ——> EP <——> E + P. These mechanisms can be divided into single-substrate and multiple-substrate mechanisms. Kinetic studies on enzymes that only bind one substrate, such as triosephosphate isomerase, aim to measure the affinity with which the enzyme binds this substrate and the turnover rate. Some other examples of enzymes are phosphofructokinase and hexokinase, both of which are important for cellular respiration (glycolysis).When enzymes bind multiple substrates, such as dihydrofolate reductase (shown right), enzyme kinetics can also show the sequence in which these substrates bind and the sequence in which products are released. An example of enzymes that bind a single substrate and release multiple products are proteases, which cleave one protein substrate into two polypeptide products. Others join two substrates together, such as DNA polymerase linking a nucleotide to DNA. Although these mechanisms are often a complex series of steps, there is typically one rate-determining step that determines the overall kinetics. This rate-determining step may be a chemical reaction or a conformational change of the enzyme or substrates, such as those involved in the release of product(s) from the enzyme.Knowledge of the enzyme's structure is helpful in interpreting kinetic data. For example, the structure can suggest how substrates and products bind during catalysis; what changes occur during the reaction; and even the role of particular amino acid residues in the mechanism. Some enzymes change shape significantly during the mechanism; in such cases, it is helpful to determine the enzyme structure with and without bound substrate analogues that do not undergo the enzymatic reaction.Not all biological catalysts are protein enzymes; RNA-based catalysts such as ribozymes and ribosomes are essential to many cellular functions, such as RNA splicing and translation. The main difference between ribozymes and enzymes is that RNA catalysts are composed of nucleotides, whereas enzymes are composed of amino acids. Ribozymes also perform a more limited set of reactions, although their reaction mechanisms and kinetics can be analysed and classified by the same methods.