Slide 1
... Enzymes are present in all living cells, where they help converting nutrients into energy and fresh cell material. Enzymes breakdown of food materials into simpler compounds. Examples: - pepsin, trypsin and peptidases break down proteins into amino acids - lipases split fats into glycerol and fatty ...
... Enzymes are present in all living cells, where they help converting nutrients into energy and fresh cell material. Enzymes breakdown of food materials into simpler compounds. Examples: - pepsin, trypsin and peptidases break down proteins into amino acids - lipases split fats into glycerol and fatty ...
Chapter Eight: Enzymes: Basic Concepts and Kinetics
... VMAX is altered. In mixed inhibition both values may be altered. 42. What are transition state analogs? Answer: These potent inhibitors mimic the structure of the substrate during the transition state of the catalytic process. They bind very tightly to the catalytic site and are useful in determinin ...
... VMAX is altered. In mixed inhibition both values may be altered. 42. What are transition state analogs? Answer: These potent inhibitors mimic the structure of the substrate during the transition state of the catalytic process. They bind very tightly to the catalytic site and are useful in determinin ...
Investigating Catalase - churchillcollegebiblio
... Cut a small (1cm-ish X 1cm-ish) square out of filter paper. Using forceps, dip the filter paper square into the enzyme solution, then remove it and drain it on a paper towel. Pour hydrogen peroxide into the plastic vial. Using the forceps, put the filter paper disc into the bottom of the vial of hyd ...
... Cut a small (1cm-ish X 1cm-ish) square out of filter paper. Using forceps, dip the filter paper square into the enzyme solution, then remove it and drain it on a paper towel. Pour hydrogen peroxide into the plastic vial. Using the forceps, put the filter paper disc into the bottom of the vial of hyd ...
Intro metabolism
... must always "balance" a chemical equation by having an equal number of atoms of each element on both sides of the arrow. ...
... must always "balance" a chemical equation by having an equal number of atoms of each element on both sides of the arrow. ...
enzymes - Moodle
... molecules will no longer fit in it At pH values slightly different from the enzyme’s optimum value, small changes in the charges of the enzyme and it’s substrate molecules will occur This change in ionisation will affect the binding of the substrate with the active site. ...
... molecules will no longer fit in it At pH values slightly different from the enzyme’s optimum value, small changes in the charges of the enzyme and it’s substrate molecules will occur This change in ionisation will affect the binding of the substrate with the active site. ...
ENZYMES A protein with catalytic properties due to its
... molecules will no longer fit in it At pH values slightly different from the enzyme’s optimum value, small changes in the charges of the enzyme and it’s substrate molecules will occur This change in ionisation will affect the binding of the substrate with the active site. ...
... molecules will no longer fit in it At pH values slightly different from the enzyme’s optimum value, small changes in the charges of the enzyme and it’s substrate molecules will occur This change in ionisation will affect the binding of the substrate with the active site. ...
Catalytic Strategies
... Basic Catalytic Principles • What are the four strategies used by enzymes to carry out catalysis? – covalent catalysis • functional groups in enzyme act as nucleophile – OH groups of serine – SH groups of cysteine – Imidazole group of histidine ...
... Basic Catalytic Principles • What are the four strategies used by enzymes to carry out catalysis? – covalent catalysis • functional groups in enzyme act as nucleophile – OH groups of serine – SH groups of cysteine – Imidazole group of histidine ...
Ch 6 Notes: Metabolism: Sum of all chemical reactions in an organism
... ATP is a coupled reaction. It is partnered with other reactions, one reaction (ATP) gives energy (exergonic), the other reaction takes energy (endergonic reaction) to do work. The energy to anabolize ADP back to ATP comes from light (in plants) or from food ...
... ATP is a coupled reaction. It is partnered with other reactions, one reaction (ATP) gives energy (exergonic), the other reaction takes energy (endergonic reaction) to do work. The energy to anabolize ADP back to ATP comes from light (in plants) or from food ...
Enzymes
... What is their function? Give an example of a specific enzyme. What happens to the rate of an enzymecatalysed reaction as temperature increases? What is the name of the model used to describe how enzymes work? What happens to enzymes as temperature or pH increases? What effect does this have on the r ...
... What is their function? Give an example of a specific enzyme. What happens to the rate of an enzymecatalysed reaction as temperature increases? What is the name of the model used to describe how enzymes work? What happens to enzymes as temperature or pH increases? What effect does this have on the r ...
Catalytic Strategies I
... a. Compares the rates of reactions one could perform without enzymes in a test tube with biological reaction rates using the enzymatic approach III. What role does Transition State stabilization play in enzyme catalysis [S4] a. There is a free energy to create a product known as DELTA-G. b. You have ...
... a. Compares the rates of reactions one could perform without enzymes in a test tube with biological reaction rates using the enzymatic approach III. What role does Transition State stabilization play in enzyme catalysis [S4] a. There is a free energy to create a product known as DELTA-G. b. You have ...
Enzyme Inhibition
... When the inhibitor is present it fits into its site and there is a conformational change in the enzyme molecule The enzyme’s molecular shape changes The active site of the substrate changes The substrate cannot bind with the substrate ...
... When the inhibitor is present it fits into its site and there is a conformational change in the enzyme molecule The enzyme’s molecular shape changes The active site of the substrate changes The substrate cannot bind with the substrate ...
Enzymes - fblocks
... 1- All Enzymes are Proteins (except ribozymes that are RNA) 2- Active Site Each enzyme molecules contain a special pocket called the active site. The substrate bind with the active site to form enzyme substrate-complex (ES) which is converted to enzyme-product complex (EP). EP dissociates to enzyme ...
... 1- All Enzymes are Proteins (except ribozymes that are RNA) 2- Active Site Each enzyme molecules contain a special pocket called the active site. The substrate bind with the active site to form enzyme substrate-complex (ES) which is converted to enzyme-product complex (EP). EP dissociates to enzyme ...
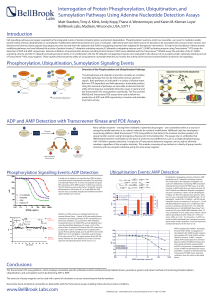
Interrogation of Protein Phosphorylation
... protein states, whereas ubiquitination or sumoylation modifications determine protein turn-over or transport. Aberrant kinases have been known for decades to be associated with various human cancers and diseases and have thus been popular drug targets, but only recently have the ubiquitin-and SUMO-c ...
... protein states, whereas ubiquitination or sumoylation modifications determine protein turn-over or transport. Aberrant kinases have been known for decades to be associated with various human cancers and diseases and have thus been popular drug targets, but only recently have the ubiquitin-and SUMO-c ...
Amino Acids Proteins, and Enzymes
... What are amino acids? Amino acids • are the building blocks of proteins. • contain a carboxylic acid group and an amino group on the alpha () carbon. • are ionized in solution. • each contain a different side group (R). ...
... What are amino acids? Amino acids • are the building blocks of proteins. • contain a carboxylic acid group and an amino group on the alpha () carbon. • are ionized in solution. • each contain a different side group (R). ...
enzymes
... more rarely, of its tetrahydrobiopterin cofactor. Phenylalanine accumulates in all body fluids because it cannot be converted into tyrosine. Normally, three-quarters of the phenylalanine is converted into tyrosine, and the other quarter becomes incorporated into proteins. The accumulation of phenylp ...
... more rarely, of its tetrahydrobiopterin cofactor. Phenylalanine accumulates in all body fluids because it cannot be converted into tyrosine. Normally, three-quarters of the phenylalanine is converted into tyrosine, and the other quarter becomes incorporated into proteins. The accumulation of phenylp ...
Enzymes: the little molecules that could
... used, proteases have time to work before kneading, making the dough easier to knead. ...
... used, proteases have time to work before kneading, making the dough easier to knead. ...
What are enzymes and how do they work
... 13. a. Is the relative position of a specific R-group within an active site important? Yes b. Which mutant helps to answer this question? Mut1 14. When the transfer of a proton between the enzyme and substrate is prevented, is the reaction rate changed slightly or dramatically? dramatically 15. Even ...
... 13. a. Is the relative position of a specific R-group within an active site important? Yes b. Which mutant helps to answer this question? Mut1 14. When the transfer of a proton between the enzyme and substrate is prevented, is the reaction rate changed slightly or dramatically? dramatically 15. Even ...
KTH | KD1500 Physical Biochemistry 7.5 credits
... these processes can be described with the laws of thermodynamics. From given conditions the course of action can be predicted. In this course we will go through the concepts and put them in a biological perspectiv, we show how biologival and biochemical processes can be understood from basic physica ...
... these processes can be described with the laws of thermodynamics. From given conditions the course of action can be predicted. In this course we will go through the concepts and put them in a biological perspectiv, we show how biologival and biochemical processes can be understood from basic physica ...
The following Text was taken from the Student Lab Manual for
... fashion. The two primary classes of enzyme inhibitors are called competitive and noncompetitive inhibitors. Competitive inhibitors are molecules that decrease an enzyme’s activity by binding to the active site of the enzyme and preventing binding of the enzyme’s normal substrate. Competitive inhibit ...
... fashion. The two primary classes of enzyme inhibitors are called competitive and noncompetitive inhibitors. Competitive inhibitors are molecules that decrease an enzyme’s activity by binding to the active site of the enzyme and preventing binding of the enzyme’s normal substrate. Competitive inhibit ...
here - My Haiku
... 28. List 4 ways to control an enzymatic reaction a. Inhibitors b. Activators c. Change in pH d. Change in temperature 29. What has happened if an enzyme is denatured? Why will it no longer work? If an enzyme is denatured, it loses its shape. This causes the active site to no longer be the correct sh ...
... 28. List 4 ways to control an enzymatic reaction a. Inhibitors b. Activators c. Change in pH d. Change in temperature 29. What has happened if an enzyme is denatured? Why will it no longer work? If an enzyme is denatured, it loses its shape. This causes the active site to no longer be the correct sh ...
National 4- Production of cheese
... a. The variety of protein shapes and functions arises from the sequence of amino acids. b. Functions of proteins to include structural, enzymes, hormones, antibodies and receptors. c. Enzymes function as biological catalysts and are made by all living cells. They speed up cellular reactions and are ...
... a. The variety of protein shapes and functions arises from the sequence of amino acids. b. Functions of proteins to include structural, enzymes, hormones, antibodies and receptors. c. Enzymes function as biological catalysts and are made by all living cells. They speed up cellular reactions and are ...
Protease - etcsciencestudents
... enzyme isn’t present, the lactose cannot be converted into sugars such as glucose. A lack of this enzyme causes lactose intolerance. The lactose can’t be broken down and acts as a great food source ...
... enzyme isn’t present, the lactose cannot be converted into sugars such as glucose. A lack of this enzyme causes lactose intolerance. The lactose can’t be broken down and acts as a great food source ...
Enzyme
... • Reaction rate increases as enzyme concentration increase • (at constant and very high substrate concentration) • At higher enzyme concentration more substrate will bind with enzyme ...
... • Reaction rate increases as enzyme concentration increase • (at constant and very high substrate concentration) • At higher enzyme concentration more substrate will bind with enzyme ...
Enzyme kinetics

Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or an agonist might inhibit the enzyme.Enzymes are usually protein molecules that manipulate other molecules — the enzymes' substrates. These target molecules bind to an enzyme's active site and are transformed into products through a series of steps known as the enzymatic mechanismE + S <——> ES <——> ES*< ——> EP <——> E + P. These mechanisms can be divided into single-substrate and multiple-substrate mechanisms. Kinetic studies on enzymes that only bind one substrate, such as triosephosphate isomerase, aim to measure the affinity with which the enzyme binds this substrate and the turnover rate. Some other examples of enzymes are phosphofructokinase and hexokinase, both of which are important for cellular respiration (glycolysis).When enzymes bind multiple substrates, such as dihydrofolate reductase (shown right), enzyme kinetics can also show the sequence in which these substrates bind and the sequence in which products are released. An example of enzymes that bind a single substrate and release multiple products are proteases, which cleave one protein substrate into two polypeptide products. Others join two substrates together, such as DNA polymerase linking a nucleotide to DNA. Although these mechanisms are often a complex series of steps, there is typically one rate-determining step that determines the overall kinetics. This rate-determining step may be a chemical reaction or a conformational change of the enzyme or substrates, such as those involved in the release of product(s) from the enzyme.Knowledge of the enzyme's structure is helpful in interpreting kinetic data. For example, the structure can suggest how substrates and products bind during catalysis; what changes occur during the reaction; and even the role of particular amino acid residues in the mechanism. Some enzymes change shape significantly during the mechanism; in such cases, it is helpful to determine the enzyme structure with and without bound substrate analogues that do not undergo the enzymatic reaction.Not all biological catalysts are protein enzymes; RNA-based catalysts such as ribozymes and ribosomes are essential to many cellular functions, such as RNA splicing and translation. The main difference between ribozymes and enzymes is that RNA catalysts are composed of nucleotides, whereas enzymes are composed of amino acids. Ribozymes also perform a more limited set of reactions, although their reaction mechanisms and kinetics can be analysed and classified by the same methods.