Enzyme
... When the active site is prevented from combining with the substrate, INHIBITION occurs This can be a very effective way of controlling reaction rates. In some reactions, the PRODUCT competes with the substrate. High Product means lower reaction rate! In a Metabolic Pathway, The end product may be su ...
... When the active site is prevented from combining with the substrate, INHIBITION occurs This can be a very effective way of controlling reaction rates. In some reactions, the PRODUCT competes with the substrate. High Product means lower reaction rate! In a Metabolic Pathway, The end product may be su ...
Document
... a. Increase in substrate or enzyme concentration will increase the rate b. At some substrate concentrations, the active sites on all enzymes are engaged and has reached saturation 2. Temperature a. Increase in temperature, increases the rate of enzymatic reactions by increasing kinetic energy of mol ...
... a. Increase in substrate or enzyme concentration will increase the rate b. At some substrate concentrations, the active sites on all enzymes are engaged and has reached saturation 2. Temperature a. Increase in temperature, increases the rate of enzymatic reactions by increasing kinetic energy of mol ...
Energy Concepts
... 1. Each enzyme binds a specific reactant molecule (substrate) at its active site. 2. After the reactants are converted, the products are released and the enzyme remains unchanged. 3. Enzyme + Substrate Enzyme-substrate complex Product + Enzyme vi. Factors Affecting Enzyme Activity 1. Concentrati ...
... 1. Each enzyme binds a specific reactant molecule (substrate) at its active site. 2. After the reactants are converted, the products are released and the enzyme remains unchanged. 3. Enzyme + Substrate Enzyme-substrate complex Product + Enzyme vi. Factors Affecting Enzyme Activity 1. Concentrati ...
Structure and Mechanism of Chymotrypsin
... of chymotrypsin is probably understood in more detail than any other enzyme a t the present time. As enzymes go, it is a very simple one, and it has proved accessible t o study by a wide range of different techniques. Although it is 7 years since the first molecular model of chymotrypsin was built,l ...
... of chymotrypsin is probably understood in more detail than any other enzyme a t the present time. As enzymes go, it is a very simple one, and it has proved accessible t o study by a wide range of different techniques. Although it is 7 years since the first molecular model of chymotrypsin was built,l ...
Industrial enzyme production
... • Replace acids in the starch processing industry and alkalis or oxidizing agents in fabric desizing; • Reduce the use of sulfide in tanneries; • Replace pumice stones for “stonewashing” jeans; • Allow for more complete digestion of animal feed leading to less animal waste; and • Remove stains from ...
... • Replace acids in the starch processing industry and alkalis or oxidizing agents in fabric desizing; • Reduce the use of sulfide in tanneries; • Replace pumice stones for “stonewashing” jeans; • Allow for more complete digestion of animal feed leading to less animal waste; and • Remove stains from ...
Enzyme Lab - marric.us
... pH is in the range of under 7, a solution is said to be acidic; if the pH is 7, the solution is neutral: and if the pH is in the range of over 7, the solution is basic. ..Amino acid side chains contain groups such as -COOH and -NH2, that readily gain or lose H+ ions. ..As the pH is lowered an enzyme ...
... pH is in the range of under 7, a solution is said to be acidic; if the pH is 7, the solution is neutral: and if the pH is in the range of over 7, the solution is basic. ..Amino acid side chains contain groups such as -COOH and -NH2, that readily gain or lose H+ ions. ..As the pH is lowered an enzyme ...
World record enzymes
... One vital class of proteins is enzymes, which are catalysts, i.e. they speed up chemical reactions without being consumed in the process. Without them, many reactions essential for life would be far too slow for life to exist. Catalysts do not affect the equilibrium of reactions, only the rate at wh ...
... One vital class of proteins is enzymes, which are catalysts, i.e. they speed up chemical reactions without being consumed in the process. Without them, many reactions essential for life would be far too slow for life to exist. Catalysts do not affect the equilibrium of reactions, only the rate at wh ...
Biochemistry - cloudfront.net
... Lipids tend to be the largest of the organic molecules. They carry more energy then carbohydrates, but they are not utilized to make ATP during respiration like glucose because they are too difficult and too large to ...
... Lipids tend to be the largest of the organic molecules. They carry more energy then carbohydrates, but they are not utilized to make ATP during respiration like glucose because they are too difficult and too large to ...
Training Question 3: Rubric
... Core concept learning objective: 1b. Given a description of a known regulatory molecule, students should be able to predict how pathway(s) would respond to changes in regulator levels. Bloom’s level: a) Level 2 comprehension b) Level 3 application High proficiency (3) Answer should have made three p ...
... Core concept learning objective: 1b. Given a description of a known regulatory molecule, students should be able to predict how pathway(s) would respond to changes in regulator levels. Bloom’s level: a) Level 2 comprehension b) Level 3 application High proficiency (3) Answer should have made three p ...
Document
... • Reconstructions (e.g. Delaye et al. OLEB in press) cannot reach deep enough • The fact that metabolic enzymes are not well conserved does not mean that they were not there! • Scaffolds (pre-RNA, primitive metabolic reactions) may have disappeared without leaving a trace behind!!! • A more syntheti ...
... • Reconstructions (e.g. Delaye et al. OLEB in press) cannot reach deep enough • The fact that metabolic enzymes are not well conserved does not mean that they were not there! • Scaffolds (pre-RNA, primitive metabolic reactions) may have disappeared without leaving a trace behind!!! • A more syntheti ...
Name
... know. Do you know what Jell-O is really made from? Are you ready? That sweet colorful treat is actually made out of hides, bones, and inedible connecting tissue from animals butchered for meat. No? Yup! All gelatin is made out of discarded animal parts — the tough parts: bone and skin. Moreover, all ...
... know. Do you know what Jell-O is really made from? Are you ready? That sweet colorful treat is actually made out of hides, bones, and inedible connecting tissue from animals butchered for meat. No? Yup! All gelatin is made out of discarded animal parts — the tough parts: bone and skin. Moreover, all ...
Mutation
... Use Computer modeling to predict where mutation is preferred Test stability/activity/stability of enzyme in vivo/in vitro ...
... Use Computer modeling to predict where mutation is preferred Test stability/activity/stability of enzyme in vivo/in vitro ...
Bioenergetics Study Guide Bioenergetics – Overview Use the
... Electron Transport Chain - What is it? - How does it work? (General overview – You will not need to have all the steps memorized, but be able to describe the steps if shown a diagram.) - What is used during glycolysis? - What is produced? - How are the electron transport chain and the citric acid cy ...
... Electron Transport Chain - What is it? - How does it work? (General overview – You will not need to have all the steps memorized, but be able to describe the steps if shown a diagram.) - What is used during glycolysis? - What is produced? - How are the electron transport chain and the citric acid cy ...
How to write an effective conclusion
... As the temperature increases the molecules have greater kinetic energy. This increases the number of collisions thereby increasing the rate of reaction. As the temperature continues increasing the rate of reaction decreases after the optimum temperature. This is because the enzyme, which is a protei ...
... As the temperature increases the molecules have greater kinetic energy. This increases the number of collisions thereby increasing the rate of reaction. As the temperature continues increasing the rate of reaction decreases after the optimum temperature. This is because the enzyme, which is a protei ...
Catalase Design Lab - Westminster Public Schools Wiki
... peroxide. Hydrogen peroxide is a toxic waste product that is part of your cell’s metabolism. Catalase converts H2O2 to harmless water and oxygen gas. As with any enzyme, how well catalase works is dependent on its environment – the acidity, the amount of substrate, the amount of enzyme, salt concent ...
... peroxide. Hydrogen peroxide is a toxic waste product that is part of your cell’s metabolism. Catalase converts H2O2 to harmless water and oxygen gas. As with any enzyme, how well catalase works is dependent on its environment – the acidity, the amount of substrate, the amount of enzyme, salt concent ...
ENZYME
... are said to have complete or true cellulases. The majority of organisms that produce cellulases can only hydrolyze the cellulose in their diets to certain extent. they are known as incomplete cellulases. These cellulases unable to digest cellulose exhaustively can still generate sufficient amoun ...
... are said to have complete or true cellulases. The majority of organisms that produce cellulases can only hydrolyze the cellulose in their diets to certain extent. they are known as incomplete cellulases. These cellulases unable to digest cellulose exhaustively can still generate sufficient amoun ...
Artificial enzymes
... Schematic diagram of the molecular imprinting process: (i) the template is mixed with vinyl monomers, selected to interact with specific functionality of the template, (ii) the templatemonomer complex may be formed by covalent or non-covalent associations (or a mixture of both), (iii) the complex is ...
... Schematic diagram of the molecular imprinting process: (i) the template is mixed with vinyl monomers, selected to interact with specific functionality of the template, (ii) the templatemonomer complex may be formed by covalent or non-covalent associations (or a mixture of both), (iii) the complex is ...
... In this Appendix there are 3 art@ies. These articles provide a basic understanding of the mechanism of how HCA works. The first article is by John Lowenstein of Brandeis University who was one of the key figures in the early research conductad on HCA. The article entitled “Experiments with Hydroxyci ...
Enzymes Lab
... Enzymes Lab I. Introduction A. Enzyme definition 1. Protein – composed of amino acids 2. Catalytic- increase rate of a reaction B. Mechanism of action 1. Based on shape 2. E + S ES complex E + P 3. “Lock and Key” 4. Very specific 5. Decrease in energy of activation II. Experimental design A. Back ...
... Enzymes Lab I. Introduction A. Enzyme definition 1. Protein – composed of amino acids 2. Catalytic- increase rate of a reaction B. Mechanism of action 1. Based on shape 2. E + S ES complex E + P 3. “Lock and Key” 4. Very specific 5. Decrease in energy of activation II. Experimental design A. Back ...
Types of Reactions and Equilibrium
... Describe the relationship between chemical bonds and chemical reactions. How is energy involved? ...
... Describe the relationship between chemical bonds and chemical reactions. How is energy involved? ...
Enzyme. Kinetics Mechanisms of enzyme action Specificity and
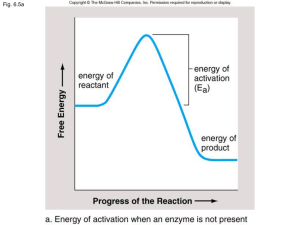
... chemical reaction by reducing an activation energy, and which is left unchanged by the reaction. For two molecules to react they must collide with one another. They must collide in the right direction (orientation) and with sufficient energy. The sufficient energy means that in them they have enough ...
... chemical reaction by reducing an activation energy, and which is left unchanged by the reaction. For two molecules to react they must collide with one another. They must collide in the right direction (orientation) and with sufficient energy. The sufficient energy means that in them they have enough ...
1/(V/Km)
... v = k2[Et][S] / ((k-1 + k2)/k1 + [S]) = V[S] / (Km + [S]) where V = k2[Et] and is thus a function of total enzyme concentration, and Km = (k-1 + k2)/k1 is derived from multiple rate constants. The complexity of this term increases for more complicated kinetic mechanisms. The value of Km is taken as ...
... v = k2[Et][S] / ((k-1 + k2)/k1 + [S]) = V[S] / (Km + [S]) where V = k2[Et] and is thus a function of total enzyme concentration, and Km = (k-1 + k2)/k1 is derived from multiple rate constants. The complexity of this term increases for more complicated kinetic mechanisms. The value of Km is taken as ...
Reversible Competitive Inhibitor
... Mathematically, the effect of a competitive inhibitor on an enzyme is to decrease the affinity of an enzyme for a substrate. The affinity is measured by Km, the Michaelis constant, and a lower affinity appears as a higher value for Km in the presence of the inhibitor. Vmax, however, is unchanged, bu ...
... Mathematically, the effect of a competitive inhibitor on an enzyme is to decrease the affinity of an enzyme for a substrate. The affinity is measured by Km, the Michaelis constant, and a lower affinity appears as a higher value for Km in the presence of the inhibitor. Vmax, however, is unchanged, bu ...
Enzyme kinetics

Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or an agonist might inhibit the enzyme.Enzymes are usually protein molecules that manipulate other molecules — the enzymes' substrates. These target molecules bind to an enzyme's active site and are transformed into products through a series of steps known as the enzymatic mechanismE + S <——> ES <——> ES*< ——> EP <——> E + P. These mechanisms can be divided into single-substrate and multiple-substrate mechanisms. Kinetic studies on enzymes that only bind one substrate, such as triosephosphate isomerase, aim to measure the affinity with which the enzyme binds this substrate and the turnover rate. Some other examples of enzymes are phosphofructokinase and hexokinase, both of which are important for cellular respiration (glycolysis).When enzymes bind multiple substrates, such as dihydrofolate reductase (shown right), enzyme kinetics can also show the sequence in which these substrates bind and the sequence in which products are released. An example of enzymes that bind a single substrate and release multiple products are proteases, which cleave one protein substrate into two polypeptide products. Others join two substrates together, such as DNA polymerase linking a nucleotide to DNA. Although these mechanisms are often a complex series of steps, there is typically one rate-determining step that determines the overall kinetics. This rate-determining step may be a chemical reaction or a conformational change of the enzyme or substrates, such as those involved in the release of product(s) from the enzyme.Knowledge of the enzyme's structure is helpful in interpreting kinetic data. For example, the structure can suggest how substrates and products bind during catalysis; what changes occur during the reaction; and even the role of particular amino acid residues in the mechanism. Some enzymes change shape significantly during the mechanism; in such cases, it is helpful to determine the enzyme structure with and without bound substrate analogues that do not undergo the enzymatic reaction.Not all biological catalysts are protein enzymes; RNA-based catalysts such as ribozymes and ribosomes are essential to many cellular functions, such as RNA splicing and translation. The main difference between ribozymes and enzymes is that RNA catalysts are composed of nucleotides, whereas enzymes are composed of amino acids. Ribozymes also perform a more limited set of reactions, although their reaction mechanisms and kinetics can be analysed and classified by the same methods.